IgA
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
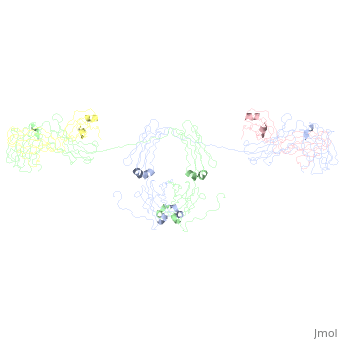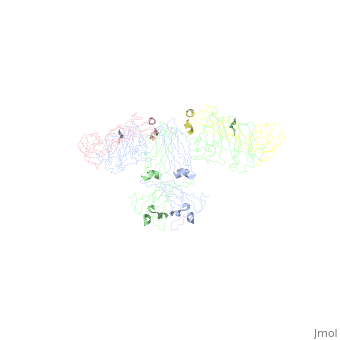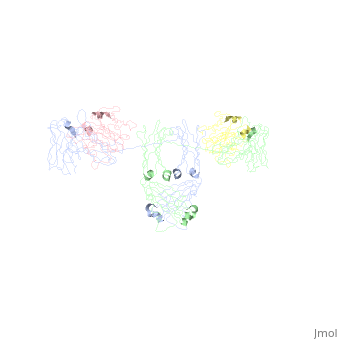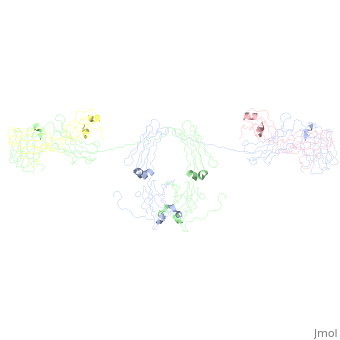
| - | <StructureSection load='1iga' size='450' side='right' scene='' caption=''> | + | <StructureSection load='1iga' size='450' side='right' scene='' caption='Model of human Iga1 [[1iga]]'> |
| + | |||
== Introduction to IgA == | == Introduction to IgA == | ||
The most extensive surface in contact with the external environment is not our skin, but the epithelial lining of our gastrointestinal, respiratory, and urogenital tracts <ref name="seven">PMID:17428798</ref>. As a first line of defense in maintenance the integrity our mucosa, the immune system manufactures and secretes dimeric IgA to neutralize pathogenic organisms <ref name="five">PMID:15111057</ref> and exclude the entry of commensals at the mucosal border <ref name="nineseven">PMID:19079336</ref>. In the serum, IgA functions as a second line of defense against pathogens that may breech the epithelial boundary <ref name="five" />. The body produces more IgA than any other antibody isotype <ref name="nineseven"/>. In fact, IgA is the most abundant antibody in the body, further illustrating IgA's critical role in immunity <ref name="ten">PMID:10064707</ref>. | The most extensive surface in contact with the external environment is not our skin, but the epithelial lining of our gastrointestinal, respiratory, and urogenital tracts <ref name="seven">PMID:17428798</ref>. As a first line of defense in maintenance the integrity our mucosa, the immune system manufactures and secretes dimeric IgA to neutralize pathogenic organisms <ref name="five">PMID:15111057</ref> and exclude the entry of commensals at the mucosal border <ref name="nineseven">PMID:19079336</ref>. In the serum, IgA functions as a second line of defense against pathogens that may breech the epithelial boundary <ref name="five" />. The body produces more IgA than any other antibody isotype <ref name="nineseven"/>. In fact, IgA is the most abundant antibody in the body, further illustrating IgA's critical role in immunity <ref name="ten">PMID:10064707</ref>. | ||
| Line 16: | Line 17: | ||
'''Fab and Fc fragments''' | '''Fab and Fc fragments''' | ||
| - | :Another common way of describing antibody structure is in terms of its Fab and Fc fragments. Each light chains are composed of 2 immunoglobulin domains: one variable domain | + | :Another common way of describing antibody structure is in terms of its Fab and Fc fragments. Each light chains are composed of 2 immunoglobulin domains: one variable domain and one constant domain. Heavy chains composed of 4 Ig domains: one V-type and 3 C-type, named CH1 - CH3. A linking hinge region separates the CH2 and CH3 domains. Proteolytic cleavage at the hinge region by the protease papain, or a similar protease, yields 2 Fab fragments and 1 Fc fragment. Each <scene name='Rebecca_Martin/Sandbox1/Fab_ex/1'>Fab fragment</scene> contains 2 variable domains, one from the heavy chain and one from the light chain, and 2 constant domains one from the light chain and the Ch1 domain from the heavy chain. The <scene name='Rebecca_Martin/Sandbox1/Fc/1'>Fc fragment</scene> Fc fragment contains 4 constant domains: the Ch2 and Ch3 domains from each of the heavy chains. Since the variable portions determine antigen specificity, the Fab fragments are generally thought of as the antigen-binding portion. The Fc fragment is important in binding various receptors, many of which are isotype specific and are named after the isotype of the ligand, i.e. FcαR binds the Fc portion of IgA. |
'''Immunoglobulin domains''' | '''Immunoglobulin domains''' | ||
| Line 36: | Line 37: | ||
:These data must be taken into account with other hinge region characteristics <ref name="five"/>. IgA1’s hinge region contains 5 sites of O-glycosylation, while IgA2’s hinge region contains none. In addition, IgA1’s hinge region contains 10 Pro residues, while IgA2’s region contains 6. In comparison, IgG’s hinge region contains No glycine residues reside in the hinge regions of either IgA1 or IgA2. The presence of prolines, the absence of glycine and the presence of glycosylated residues in IgA1 all amount to '''increased hinge rigidity''' in comparison to IgG1. | :These data must be taken into account with other hinge region characteristics <ref name="five"/>. IgA1’s hinge region contains 5 sites of O-glycosylation, while IgA2’s hinge region contains none. In addition, IgA1’s hinge region contains 10 Pro residues, while IgA2’s region contains 6. In comparison, IgG’s hinge region contains No glycine residues reside in the hinge regions of either IgA1 or IgA2. The presence of prolines, the absence of glycine and the presence of glycosylated residues in IgA1 all amount to '''increased hinge rigidity''' in comparison to IgG1. | ||
| - | + | ||
'''N-glycosylation''' | '''N-glycosylation''' | ||
:In the harsh mucosal environment, glycosylated residues protect the protein from proteases <ref name="five"/>. Both IgA1 and IgA2 display N-glycosylated residues. IgA1 has 3, at N263 on beta strand B on the Ch2 chain and on the J tail at N459. In IgA2, additional sites of N-glycosylation include Asn166 on the beta strand G of Ch1 and Asn337 of beta strand G on Ch2. Some alloforms of IgA2 are also N-glycosylated at Asn211 on Ch2. An increased need for protection against proteolytic cleavage at the hinge region accounts for the presence of O-glycosylation in IgA1’s hinge region, particularly cleavage by bacterial metalloproteases. The glycosylation residues provide increased steric hindrance, and creating difficulty in fitting the peptide in the protease’s active site. In comparison to IgG, which is only 2.9% (w/w) glycosylated, IgA1 is 9.5% (w/w) and IgA2 is 11% (w/w) glycosylated. Overall, IgA1 is more susceptible to proteases than IgA2. | :In the harsh mucosal environment, glycosylated residues protect the protein from proteases <ref name="five"/>. Both IgA1 and IgA2 display N-glycosylated residues. IgA1 has 3, at N263 on beta strand B on the Ch2 chain and on the J tail at N459. In IgA2, additional sites of N-glycosylation include Asn166 on the beta strand G of Ch1 and Asn337 of beta strand G on Ch2. Some alloforms of IgA2 are also N-glycosylated at Asn211 on Ch2. An increased need for protection against proteolytic cleavage at the hinge region accounts for the presence of O-glycosylation in IgA1’s hinge region, particularly cleavage by bacterial metalloproteases. The glycosylation residues provide increased steric hindrance, and creating difficulty in fitting the peptide in the protease’s active site. In comparison to IgG, which is only 2.9% (w/w) glycosylated, IgA1 is 9.5% (w/w) and IgA2 is 11% (w/w) glycosylated. Overall, IgA1 is more susceptible to proteases than IgA2. | ||
| Line 106: | Line 107: | ||
==sIgA1 and sIgA2== | ==sIgA1 and sIgA2== | ||
| - | [[Image:SIgA.jpg|thumb|Adapted from Bonner, et al 2009 and Bonner, et al 2008.]] | ||
:Binding of the secretory component to the convex edge of the Fc region of dimeric IgA1 maintains <scene name='Rebecca_Martin/Sandbox1/Siga1def/1'>Secretory IgA1</scene> in a near planar conformation, <ref name="nineten" />, <ref name="eight" />. The Fc regions align end to end without overlap, and the fab fragments remain in alignment with the Fc plane. In contrast, <scene name='Rebecca_Martin/Sandbox1/Siga1/1'>Secretory IgA2</scene> fab fragments remain out of alignment with the Fc plane. Because the secretory component resides at the convex region of the Fc portion, the D1 and D5 impart steric hindrance on the fab fragments, which are forced out of alignment. Consequently, IgA2 assumes a nonplanar conformation. The longer hinge region of IgA1 allows it to maintain its planar conformation. | :Binding of the secretory component to the convex edge of the Fc region of dimeric IgA1 maintains <scene name='Rebecca_Martin/Sandbox1/Siga1def/1'>Secretory IgA1</scene> in a near planar conformation, <ref name="nineten" />, <ref name="eight" />. The Fc regions align end to end without overlap, and the fab fragments remain in alignment with the Fc plane. In contrast, <scene name='Rebecca_Martin/Sandbox1/Siga1/1'>Secretory IgA2</scene> fab fragments remain out of alignment with the Fc plane. Because the secretory component resides at the convex region of the Fc portion, the D1 and D5 impart steric hindrance on the fab fragments, which are forced out of alignment. Consequently, IgA2 assumes a nonplanar conformation. The longer hinge region of IgA1 allows it to maintain its planar conformation. | ||
| Line 134: | Line 134: | ||
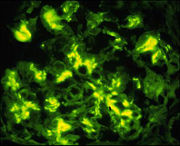
== Implications in Medicine and Science == | == Implications in Medicine and Science == | ||
[[Image:IgA_IFA.jpg|thumb|Immunofluorescence detecting IgA in IgA glomerulonephritis. From http://www.unckidneycenter.org/images/IgA_IFA.jpg, with permission]] | [[Image:IgA_IFA.jpg|thumb|Immunofluorescence detecting IgA in IgA glomerulonephritis. From http://www.unckidneycenter.org/images/IgA_IFA.jpg, with permission]] | ||
| + | |||
:IgA nephropathy is the most prevalent cause of chronic glomerulonephritis in the world and is caused by polymeric IgA1 deposited at the kidney glomeruli <ref name="eight"/>. Notably, 90% of serum IgA is IgA1, mostly in the monomeric form. The observation that individuals with IgA myeloma [http://en.wikipedia.org/wiki/Multiple_myeloma] lack nephropathy suggests an abnormality in IgA structure, leading to an abnormal amount of polymerization. Steric hindrance of the fab segments normally limits the amount of polymerization of IgA. Bonner, et al proposes that a disturbance in the hinge region or an absence of fab. Similarly, decreased O-glycosylation might could destabilize the hinge region, allowing IgA to self associate. Likewise, destabilizing this region might make IgA susceptible to cleavage of fab fragments by bacterial proteases, leading to self aggregation and renal pathology. For more information on IgA nephropathy: [http://http://www.unckidneycenter.org/contact.html]. <ref name="sn">Falk, R. "IgA Nephropathy." UNC Kidney Center, from http://www.unckidneycenter.org/kidneyhealthlibrary/iganephropathy.html.</ref>. | :IgA nephropathy is the most prevalent cause of chronic glomerulonephritis in the world and is caused by polymeric IgA1 deposited at the kidney glomeruli <ref name="eight"/>. Notably, 90% of serum IgA is IgA1, mostly in the monomeric form. The observation that individuals with IgA myeloma [http://en.wikipedia.org/wiki/Multiple_myeloma] lack nephropathy suggests an abnormality in IgA structure, leading to an abnormal amount of polymerization. Steric hindrance of the fab segments normally limits the amount of polymerization of IgA. Bonner, et al proposes that a disturbance in the hinge region or an absence of fab. Similarly, decreased O-glycosylation might could destabilize the hinge region, allowing IgA to self associate. Likewise, destabilizing this region might make IgA susceptible to cleavage of fab fragments by bacterial proteases, leading to self aggregation and renal pathology. For more information on IgA nephropathy: [http://http://www.unckidneycenter.org/contact.html]. <ref name="sn">Falk, R. "IgA Nephropathy." UNC Kidney Center, from http://www.unckidneycenter.org/kidneyhealthlibrary/iganephropathy.html.</ref>. | ||
| Line 155: | Line 156: | ||
:Crystallographic structure will yield further insights into the structure of IgA, the interactions between IgA and other molecules. | :Crystallographic structure will yield further insights into the structure of IgA, the interactions between IgA and other molecules. | ||
| - | + | </StructureSection> | |
| - | + | __NOTOC__ | |
== Links == | == Links == | ||
=== IgA === | === IgA === | ||
Current revision
| |||||||||||
Links
IgA
- Fab and Fc Fragments
- Refined crystal structure of the galactan-binding immunoglobulin fab j539 at 1.95-angstroms resolution 2fbj
- Phosphocholine binding immunoglobulin fab mc/pc603. an x-ray diffraction study at 2.7 angstroms 1mcp
- Phosphocholine binding immunoglobulin fab mc/pc603. an x-ray diffraction study at 3.1 angstroms 2mcp
- Crystal structure of human FcaRI bound to IgA1-Fc 1ow0
- Refined crystal structure of a recombinant immunoglobulin domain and a complementarity-determining region 1-grafted mutant 2imm and2imn
- Crystal structure of a Staphylococcus aureus protein (SSL7) in complex with Fc of human IgA1 2qej
- Monomeric
- Dimeric and Secretory
Related Molecules
- non-IgA antibody isotypes
- IgM: Solution structure of human Immunoglobulin M 2rcj
- IgG: Crystal structure of the intact human IgG B12 with broad and potent activity against primary HIV-1 isolates: a template for HIV vaccine design 1hzh
- IgG: Three=dimensional structure of a human immunoglobulin with a hinge deletion 1mco
- IgD: Semi-extended solution structure of human myeloma immunoglobulin D determined by constrained X-ray scattering 1zvo
- IgE: Structure of the human ige-fc bound to its high affinity receptor fc(epsilon)ri(alpha) 1f6a
- Other C-type immunoglobulin examples
- V-type immunoglobulin examples
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Bonner A, Perrier C, Corthesy B, Perkins SJ. Solution structure of human secretory component and implications for biological function. J Biol Chem. 2007 Jun 8;282(23):16969-80. Epub 2007 Apr 11. PMID:17428798 doi:http://dx.doi.org/10.1074/jbc.M701281200
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 2.9 Furtado PB, Whitty PW, Robertson A, Eaton JT, Almogren A, Kerr MA, Woof JM, Perkins SJ. Solution structure determination of monomeric human IgA2 by X-ray and neutron scattering, analytical ultracentrifugation and constrained modelling: a comparison with monomeric human IgA1. J Mol Biol. 2004 May 14;338(5):921-41. PMID:15111057 doi:http://dx.doi.org/10.1016/j.jmb.2004.03.007
- ↑ 3.0 3.1 3.2 3.3 3.4 Bonner A, Almogren A, Furtado PB, Kerr MA, Perkins SJ. Location of secretory component on the Fc edge of dimeric IgA1 reveals insight into the role of secretory IgA1 in mucosal immunity. Mucosal Immunol. 2009 Jan;2(1):74-84. Epub 2008 Oct 8. PMID:19079336 doi:http://dx.doi.org/10.1038/mi.2008.68
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 Boehm MK, Woof JM, Kerr MA, Perkins SJ. The Fab and Fc fragments of IgA1 exhibit a different arrangement from that in IgG: a study by X-ray and neutron solution scattering and homology modelling. J Mol Biol. 1999 Mar 12;286(5):1421-47. PMID:10064707 doi:http://dx.doi.org/10.1006/jmbi.1998.2556
- ↑ 5.0 5.1 5.2 5.3 Bonner A, Almogren A, Furtado PB, Kerr MA, Perkins SJ. The nonplanar secretory IgA2 and near planar secretory IgA1 solution structures rationalize their different mucosal immune responses. J Biol Chem. 2009 Feb 20;284(8):5077-87. Epub 2008 Dec 23. PMID:19109255 doi:http://dx.doi.org/10.1074/jbc.M807529200
- ↑ 6.0 6.1 6.2 6.3 Attwood, T. "Immunoglobulin superfamily " ImPrints Retrieved April, 2009, from http://www.jenner.ac.uk/Bioinformatics/ImPRINTS/immunoglobulin_superfamily_background.htm.
- ↑ (nov 22 2007). "Superfamily: immunoglobulin." SCOP, from http://scop.mrc-lmb.cam.ac.uk/scop/data/scop.b.c.b.b.html.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 Bonner A, Furtado PB, Almogren A, Kerr MA, Perkins SJ. Implications of the near-planar solution structure of human myeloma dimeric IgA1 for mucosal immunity and IgA nephropathy. J Immunol. 2008 Jan 15;180(2):1008-18. PMID:18178841
- ↑ 9.0 9.1 Herr AB, Ballister ER, Bjorkman PJ. Insights into IgA-mediated immune responses from the crystal structures of human FcalphaRI and its complex with IgA1-Fc. Nature. 2003 Jun 5;423(6940):614-20. Epub 2003 May 21. PMID:12768205 doi:http://dx.doi.org/10.1038/nature01685
- ↑ Falk, R. "IgA Nephropathy." UNC Kidney Center, from http://www.unckidneycenter.org/kidneyhealthlibrary/iganephropathy.html.
--Rebecca Martin 01:23, 2 May 2009 (IDT)
Proteopedia Page Contributors and Editors (what is this?)
Rebecca Martin, Alexander Berchansky, Michal Harel, Jaime Prilusky
 , with permission
, with permission