We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
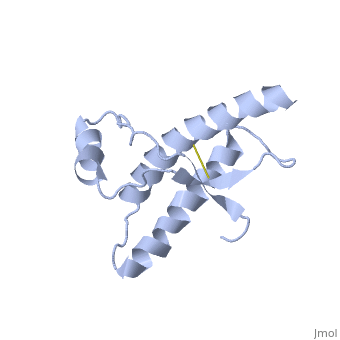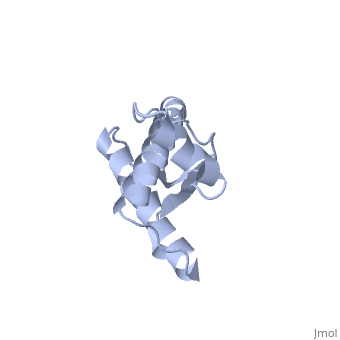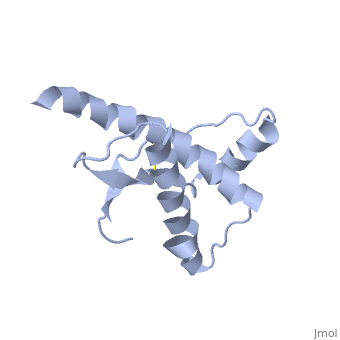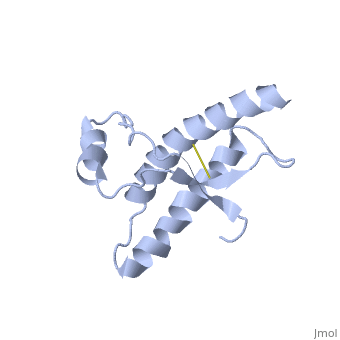
Human Prion Protein Dimer
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='1qlx' size='450' side='right' caption='' scene=''> | + | <StructureSection load='1qlx' size='450' side='right' caption='Human prion protein (PDB code [[1qlx]])' scene=''> |
== Prions as a disease causing agent== | == Prions as a disease causing agent== | ||
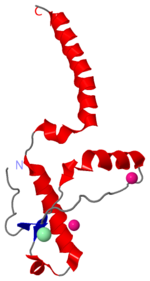
| - | [[Image:1i4m.png| | + | [[Image:1i4m.png|left|150px|thumb|Human Prion Protein in dimer form [[1i4m]]]] |
| - | + | {{clear}} | |
| - | + | ||
| - | + | ||
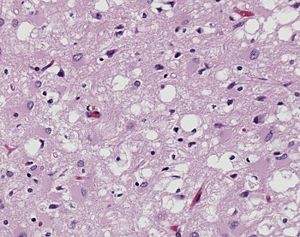
| + | [[Image:10131_lores.jpg|left|300px|thumb|Pictomicrograph of Creutzfeldt-Jakob positive brain tissue|Caption: Holes in this sponge like brain tissue result from pockets of prion aggregation <ref>[http://phil.cdc.gov/PHIL_Images/10131/10131_lores.jpg Image of Creutzfeldt-Jakob positive brain tissue] was obtained from The CDC's Public Health Image Library.</ref>]] | ||
| + | {{clear}} | ||
[http://en.wikipedia.org/wiki/Prion Prions] are infectious or genetically coded misfolded proteins which act as templates upon which properly folded prion protein monomers can aggregate. Prions contain no nucleic acid such as other infectoius molecules or organisms. Human Prion Protein or Major Prion protein, exists as a normal constituent of human cells, found mostly in the brain<ref>Centers for Disease Control and Prevention: Prions. http://www.cdc.gov/ncidod/dvrd/prions/</ref> and is called PrP<sup>C</sup>.<ref name="Prusiner">PMID:9811807</ref> PrP<sup>C</sup> is composed of mostly helix whereas the infectious form, PrP<sup>Sc</sup> (also known as "scrapie" form), is composed of high percentage beta sheets.<ref name="Prusiner">PMID:9811807</ref> | [http://en.wikipedia.org/wiki/Prion Prions] are infectious or genetically coded misfolded proteins which act as templates upon which properly folded prion protein monomers can aggregate. Prions contain no nucleic acid such as other infectoius molecules or organisms. Human Prion Protein or Major Prion protein, exists as a normal constituent of human cells, found mostly in the brain<ref>Centers for Disease Control and Prevention: Prions. http://www.cdc.gov/ncidod/dvrd/prions/</ref> and is called PrP<sup>C</sup>.<ref name="Prusiner">PMID:9811807</ref> PrP<sup>C</sup> is composed of mostly helix whereas the infectious form, PrP<sup>Sc</sup> (also known as "scrapie" form), is composed of high percentage beta sheets.<ref name="Prusiner">PMID:9811807</ref> | ||
| Line 29: | Line 29: | ||
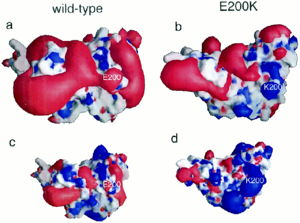
| - | [[Image:F4.large.jpg| | + | [[Image:F4.large.jpg|left|300px|thumb|Electrostatic potential alteration E200K|Caption: This shows (a&c) the electrostatic potential of wild-type Human Prion Protein with Glu200 and (b&d) the electrostatic potential of variant Lys200. <ref name="Zhang">PMID:10954699</ref>]] |
| - | + | {{clear}} | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
=== PrP<sup>Sc</sup> === | === PrP<sup>Sc</sup> === | ||
| - | |||
| - | <StructureSection load='2rnm' size='300' side='right' caption='Amyloid formation: Human Prion Protein [[2rnm]]' scene=''> | ||
The majority of this structure is <scene name='User:Erin_May/Sandbox_1/Beta_sheets/1'>beta sheets</scene>. | The majority of this structure is <scene name='User:Erin_May/Sandbox_1/Beta_sheets/1'>beta sheets</scene>. | ||
| Line 54: | Line 41: | ||
The Cystine residues which were formerly part of disulfide bonds have been reduced catalytically without any chemical reducing agent. <ref name="Knaus">PMID:11524679</ref> | The Cystine residues which were formerly part of disulfide bonds have been reduced catalytically without any chemical reducing agent. <ref name="Knaus">PMID:11524679</ref> | ||
| - | |||
| - | |||
| - | </StructureSection> | ||
===Dimer Form=== | ===Dimer Form=== | ||
| - | < | + | <scene name='Human_Prion_Protein_Dimer/Cv/1'>Major Prion Protein: Dimerized</scene> [[1i4m]]. |
The <scene name='User:Erin_May/Sandbox_1/Previously_shown_residues/1'>residues</scene>, shown above, alter the function of Major Prion Protein's ability to re-fold, however their positions on the wild-type monomer and fully unfolded PrP<sup>Sc</sup>, do not illustrate a clear mechanism for propagation. The dimer brings light to these residues' influence on the infectious qualities of PrP<sup>Sc</sup>. | The <scene name='User:Erin_May/Sandbox_1/Previously_shown_residues/1'>residues</scene>, shown above, alter the function of Major Prion Protein's ability to re-fold, however their positions on the wild-type monomer and fully unfolded PrP<sup>Sc</sup>, do not illustrate a clear mechanism for propagation. The dimer brings light to these residues' influence on the infectious qualities of PrP<sup>Sc</sup>. | ||
Current revision
| |||||||||||
Reference List
- ↑ Image of Creutzfeldt-Jakob positive brain tissue was obtained from The CDC's Public Health Image Library.
- ↑ Centers for Disease Control and Prevention: Prions. http://www.cdc.gov/ncidod/dvrd/prions/
- ↑ 3.0 3.1 Prusiner SB. Prions. Proc Natl Acad Sci U S A. 1998 Nov 10;95(23):13363-83. PMID:9811807
- ↑ Centers for Disease Control and Prevention: Bovine Spongiform Encephalopathy. http://www.cdc.gov/ncidod/dvrd/bse/index.htm
- ↑ 5.0 5.1 5.2 5.3 Lee S, Antony L, Hartmann R, Knaus KJ, Surewicz K, Surewicz WK, Yee VC. Conformational diversity in prion protein variants influences intermolecular beta-sheet formation. EMBO J. 2010 Jan 6;29(1):251-62. Epub 2009 Nov 19. PMID:19927125 doi:10.1038/emboj.2009.333
- ↑ 6.0 6.1 6.2 6.3 6.4 Knaus KJ, Morillas M, Swietnicki W, Malone M, Surewicz WK, Yee VC. Crystal structure of the human prion protein reveals a mechanism for oligomerization. Nat Struct Biol. 2001 Sep;8(9):770-4. PMID:11524679 doi:10.1038/nsb0901-770
- ↑ 7.0 7.1 7.2 7.3 Zhang Y, Swietnicki W, Zagorski MG, Surewicz WK, Sonnichsen FD. Solution structure of the E200K variant of human prion protein. Implications for the mechanism of pathogenesis in familial prion diseases. J Biol Chem. 2000 Oct 27;275(43):33650-4. PMID:10954699 doi:10.1074/jbc.C000483200
Proteopedia Page Contributors and Editors (what is this?)
Erin May, Alexander Berchansky, Jaime Prilusky, Michal Harel