Serine protease
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
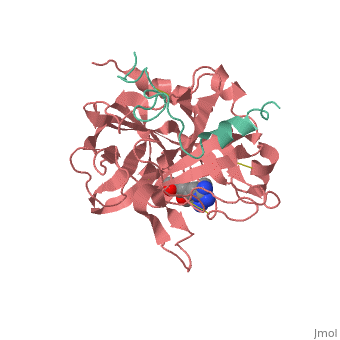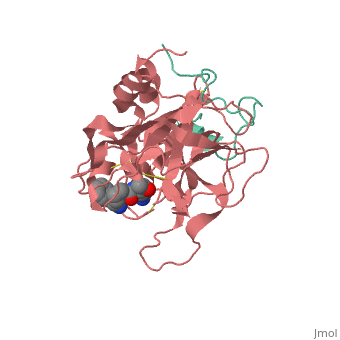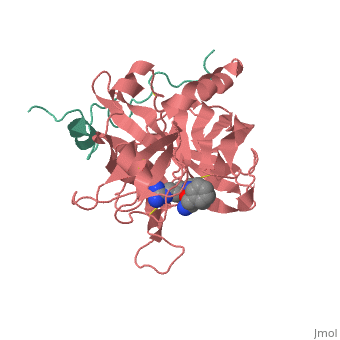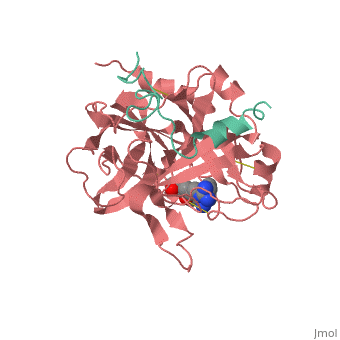
| - | <StructureSection load='1ppb' size='450' side='right' scene='' caption=' | + | <StructureSection load='1ppb' size='450' side='right' scene='' caption='Thrombin light chain (aqua) and heavy chain (red) complex with inhibitor (PDB code [[1ppb]])'> |
| - | + | ||
| - | + | ||
'''Serine proteases''', or '''proteinases''', so called due to the presence of a serine residue in the active site, are a class of enzymes that catalyse the hydrolysis of peptide bonds in proteins. | '''Serine proteases''', or '''proteinases''', so called due to the presence of a serine residue in the active site, are a class of enzymes that catalyse the hydrolysis of peptide bonds in proteins. | ||
| Line 15: | Line 13: | ||
| - | THis is <scene name='Serine_Protease/Aapk/1'>our</scene> | + | THis is <scene name='Serine_Protease/Aapk/1'>our protein fro CHEM361</scene>. |
| - | + | ==Trypsin== | |
| - | + | ||
| + | See [[Trypsin]] | ||
| - | ==Trypsin-BPTI complex== | ||
| - | The trypsin backbone is shown in pink and the trypsin inhibitor, BPTI, in yellow (PDB code [[2ptc]]). The <scene name='Serine_Protease/Active_site/3'>active site</scene> residues [Ser195-His57-Asp102-Ser214] are shown in green, the disulfide bond between residues 14-38 is shown in yellow and the Lys 15 sidechain at the specificity site in pink. | ||
==Gilman suc-AAPK-trypsin== | ==Gilman suc-AAPK-trypsin== | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Ailie McGregor, Alexander Berchansky, Ann Taylor, Harry Greenblatt, Eran Hodis, Jaime Prilusky