We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 124
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 14: | Line 14: | ||
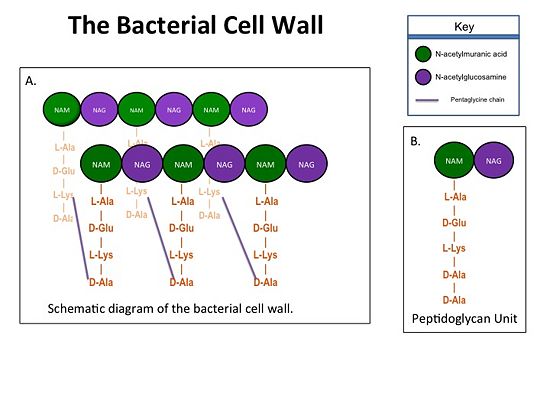
| - | '''Cell Wall Structure''' | + | ==='''Cell Wall Structure'''=== |
The cell wall, which is composed of peptidoglycans, is crucial for maintaining | The cell wall, which is composed of peptidoglycans, is crucial for maintaining | ||
the structural integrity of the bacterium. Peptidoglycans consists of | the structural integrity of the bacterium. Peptidoglycans consists of | ||
| Line 29: | Line 29: | ||
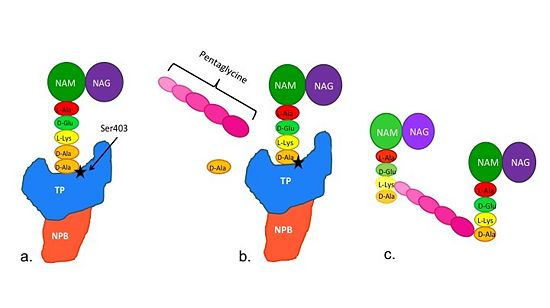
==='''Catalytic Mechanism of PBP2a'''=== | ==='''Catalytic Mechanism of PBP2a'''=== | ||
| - | [[Image:Schematic TP 3steps.jpg|thumb|alt= Alt text| |550px]] | + | [[Image:Schematic TP 3steps.jpg|thumb|alt= Alt text|Figure 2. Schematic showing Catalytic Mechanism of PBP2a |550px]] |
(a) The D-Ala-D-Ala side-chain substrate of the peptidoglycan accesses | (a) The D-Ala-D-Ala side-chain substrate of the peptidoglycan accesses | ||
the active site of the PBP2a. | the active site of the PBP2a. | ||
| Line 43: | Line 43: | ||
The entire process takes 4 milliseconds. | The entire process takes 4 milliseconds. | ||
| - | ==='''How | + | ==='''How Do Antibiotics Work?'''=== |
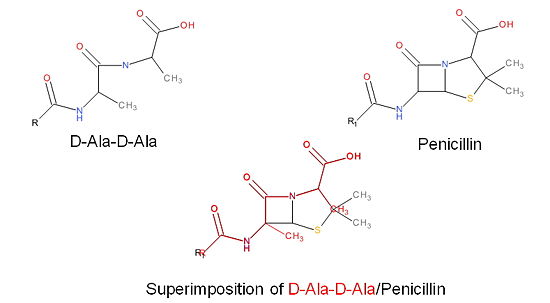
The β-lactam antibiotics inhibit bacterial growth by inhibiting PBPs and ultimately cell wall | The β-lactam antibiotics inhibit bacterial growth by inhibiting PBPs and ultimately cell wall | ||
synthesis. Specifically, β-lactams are molecular mimics of D-Ala-D-Ala, which is the normal | synthesis. Specifically, β-lactams are molecular mimics of D-Ala-D-Ala, which is the normal | ||
| Line 49: | Line 49: | ||
inhibited by the β-lactam. As a result, the synthesis of the cell wall is inhibited which leads | inhibited by the β-lactam. As a result, the synthesis of the cell wall is inhibited which leads | ||
to cell lysis. | to cell lysis. | ||
| + | [[Image:Structures on penicillin and b lactam.jpg|thumb|alt= Alt text|Figure 3. Mechanism of action of β-lactams. A. Structure of a β-lactam (penicillin) showing the amide, carboxyl, and β-lactam ring groups β-lactam ring groups. B. Structure of the D-Ala-D-Ala substrate. C. Overlay of the D-Ala-D-Ala substrate in red with penicillin demonstrating molecular mimicry.|550 px]] | ||
| + | |||
==='''PBP2a and Ceftobiprole'''=== | ==='''PBP2a and Ceftobiprole'''=== | ||
| Line 60: | Line 62: | ||
residues in PBP2a; specifically | residues in PBP2a; specifically | ||
<scene name='37/372724/Tyr446_and_met641_label/2'>Tyr446 and Met641</scene>. | <scene name='37/372724/Tyr446_and_met641_label/2'>Tyr446 and Met641</scene>. | ||
| - | As a result of | + | As a result of ceftobiprole <scene name='37/372724/R2_interaction/4'>tighter binding</scene> to PBP2a as highlighted in green , <scene name='37/372724/Ceftobiprole_in_cpk/1'>the medicine</scene>, shown as colors of the atom types ([[CPK]]), is able to more efficiently react with the serine active site residue and therefore inhibit the activity of PBP2a. |
Current revision
| |||||||||||