DOPA decarboxylase
From Proteopedia
(Difference between revisions)
| (6 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
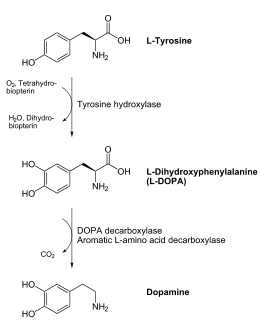
[[image:dopa.png|thumb|left|300px|'''Dopamine Synthesis''']] | [[image:dopa.png|thumb|left|300px|'''Dopamine Synthesis''']] | ||
{{Clear}} | {{Clear}} | ||
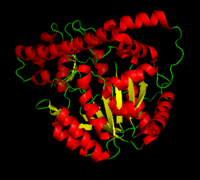
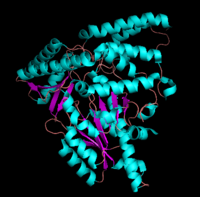
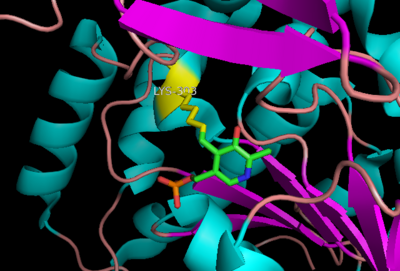
| - | '''DOPA decarboxylase''' (DDC, aromatic L-amino acid decarboxylase, tryptophan decarboxylase, 5-hydroxytryptophan decarboxylase, AAAD) ([[EC Number|EC]] 4.1.1.28) is an approximately 104 kDa protein that belongs to the '''aspartate aminotransferase family''' (fold type 1) of '''[http://en.wikipedia.org/wiki/Pyridoxal_phosphate ''PLP'']-dependent''' (vitamin B6-dependent) enzymes. The catalytically active form of the enzyme exists as a homodimer, typical of this class of enzymes.<ref name="schneider">PMID:10673430 </ref> The homodimeric form of the enzyme purified from [http://en.wikipedia.org/wiki/Sus_scrofa ''sus scrofa''] is shown in complex with the inhibitor '''[http://en.wikipedia.org/wiki/Carbidopa ''carbidopa'']''' to the right. | + | '''DOPA decarboxylase''' ('''DDC, aromatic L-amino acid decarboxylase, tryptophan decarboxylase, 5-hydroxytryptophan decarboxylase, AAAD''') ([[EC Number|EC]] 4.1.1.28) is an approximately 104 kDa protein that belongs to the '''aspartate aminotransferase family''' (fold type 1) of '''[http://en.wikipedia.org/wiki/Pyridoxal_phosphate ''PLP'']-dependent''' (vitamin B6-dependent) enzymes. The catalytically active form of the enzyme exists as a homodimer, typical of this class of enzymes.<ref name="schneider">PMID:10673430 </ref> The homodimeric form of the enzyme purified from [http://en.wikipedia.org/wiki/Sus_scrofa ''sus scrofa''] is shown in complex with the inhibitor '''[http://en.wikipedia.org/wiki/Carbidopa ''carbidopa'']''' to the right. |
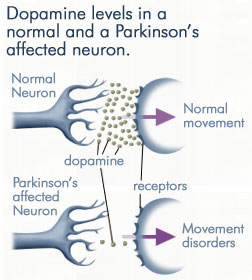
DDC catalyzes the conversion of aromatic amino acids into their corresponding amines. DOPA decarboxylase is responsible for the synthesis of '''[http://en.wikipedia.org/wiki/Dopamine ''dopamine'']''' and [http://en.wikipedia.org/wiki/Serotoninn ''serotonin''] from '''[http://en.wikipedia.org/wiki/L-dopa ''L-DOPA'']''' and [http://en.wikipedia.org/wiki/L-5-Hydroxytryptophan ''L-5-hydroxytryptophan''], respectively. It is highly stereospecific, yet relatively nonspecific in terms of substrate, making it a somewhat uninteresting enzyme to study. Although it is not typically a rate-determining step of dopamine synthesis, the decarboxylation of L-DOPA to dopamine by DDC is the controlling step for individuals with '''[http://en.wikipedia.org/wiki/Parkinson%27s_disease ''Parkinson's disease'']'''<ref name="hadjiiconstantinou">PMID:1904055 </ref> , the second most common neurodegenerative disorder, occuring in 1% of the population over the age of 65. The loss of dopaminergic neurons is the main cause of cognitive impairment and tremors observed in patients with the disease. The hallmark of the disease is the formation of [http://en.wikipedia.org/wiki/alpha-synuclein ''alpha-synuclein''] containing [http://en.wikipedia.org/wiki/Lewy_body ''Lewy bodies'']. | DDC catalyzes the conversion of aromatic amino acids into their corresponding amines. DOPA decarboxylase is responsible for the synthesis of '''[http://en.wikipedia.org/wiki/Dopamine ''dopamine'']''' and [http://en.wikipedia.org/wiki/Serotoninn ''serotonin''] from '''[http://en.wikipedia.org/wiki/L-dopa ''L-DOPA'']''' and [http://en.wikipedia.org/wiki/L-5-Hydroxytryptophan ''L-5-hydroxytryptophan''], respectively. It is highly stereospecific, yet relatively nonspecific in terms of substrate, making it a somewhat uninteresting enzyme to study. Although it is not typically a rate-determining step of dopamine synthesis, the decarboxylation of L-DOPA to dopamine by DDC is the controlling step for individuals with '''[http://en.wikipedia.org/wiki/Parkinson%27s_disease ''Parkinson's disease'']'''<ref name="hadjiiconstantinou">PMID:1904055 </ref> , the second most common neurodegenerative disorder, occuring in 1% of the population over the age of 65. The loss of dopaminergic neurons is the main cause of cognitive impairment and tremors observed in patients with the disease. The hallmark of the disease is the formation of [http://en.wikipedia.org/wiki/alpha-synuclein ''alpha-synuclein''] containing [http://en.wikipedia.org/wiki/Lewy_body ''Lewy bodies'']. | ||
| Line 14: | Line 14: | ||
{{Clear}} | {{Clear}} | ||
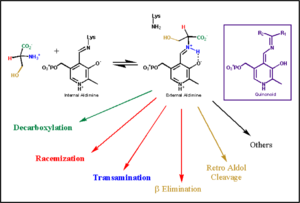
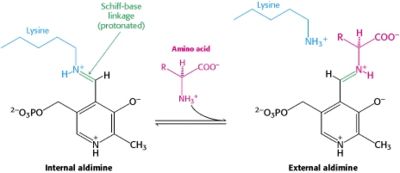
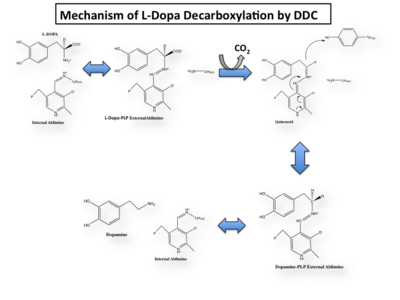
In the resting state, PLP forms a covalent bond with the amino group of the active site Lysine (internal aldimine). Upon introduction of the substrate into the active site, a new Schiff base is generated (external aldimine). This transimination step is common to '''all''' PLP-dependent enzymes. | In the resting state, PLP forms a covalent bond with the amino group of the active site Lysine (internal aldimine). Upon introduction of the substrate into the active site, a new Schiff base is generated (external aldimine). This transimination step is common to '''all''' PLP-dependent enzymes. | ||
| - | Although these enzymes have wide range of function, they can be classified into only five structural families: the '''aspartate amino transferase''', the tryptophan synthase β, the alanine racemase, the D-amino acid, and the glycogen phosphorylase. <ref name="percudani">PMID:12949584 </ref> <ref name="schneider">PMID:10673430 </ref> [[image:plp6.jpg|thumb|left|400px|'''The PLP is bound covalently to lysine residues in a Schiff base linkage (aldimine). In this form, it reacts with many free amino acids to replace the Schiff base to the lysine of the enzyme with a Schiff base to the amino acid substrate.'']] | + | Although these enzymes have wide range of function, they can be classified into only five structural families: the '''aspartate amino transferase''', the tryptophan synthase β, the alanine racemase, the D-amino acid, and the glycogen phosphorylase. <ref name="percudani">PMID:12949584 </ref> <ref name="schneider">PMID:10673430 </ref> [[image:plp6.jpg|thumb|left|400px|'''The PLP is bound covalently to lysine residues in a Schiff base linkage (aldimine). In this form, it reacts with many free amino acids to replace the Schiff base to the lysine of the enzyme with a Schiff base to the amino acid substrate.'']] |
| + | {{Clear}} | ||
---- | ---- | ||
===The Aspartate Aminotransferase Family=== | ===The Aspartate Aminotransferase Family=== | ||
[[image:aspartateamino.png|thumb|left|200px|'''Aspartate Aminotransferase''']] | [[image:aspartateamino.png|thumb|left|200px|'''Aspartate Aminotransferase''']] | ||
| + | {{Clear}} | ||
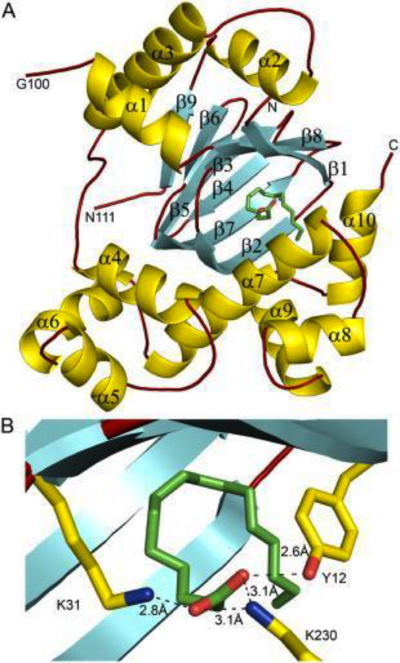
This family of PLP-dependent enzymes is also referred to as '''fold-type I''', with aspartate aminotransferase serving as the prototype. It is the most common structure of the five classes of PLP-dependent enzymes. This fold it is found in a variety of aminotransferases and decarboxylases, amongst them '''DOPA decarboxylase'''. PLP-dependent enzymes belonging to this family are catalytically active as homodimers and share a common, well-characterized structure, despite low-sequence identity. Each subunit has a large domain and a small domain. The central feature of the large domain is a seven-stranded β sheet. The small domain has either a three or four-stranded β sheet that is surrounded by α helices on one side. The cofactor PLP is covalently attached to a lysine residue in the large domain and is anchored in a way that allows the aromatic ring of PLP to pack against neighboring β strands. The active site is located in a cleft between the two domains at the interface between the two subunits. '''''Thus, enzymes of fold-type I have residues from both domains and both subunits involved in PLP-binding.''''' | This family of PLP-dependent enzymes is also referred to as '''fold-type I''', with aspartate aminotransferase serving as the prototype. It is the most common structure of the five classes of PLP-dependent enzymes. This fold it is found in a variety of aminotransferases and decarboxylases, amongst them '''DOPA decarboxylase'''. PLP-dependent enzymes belonging to this family are catalytically active as homodimers and share a common, well-characterized structure, despite low-sequence identity. Each subunit has a large domain and a small domain. The central feature of the large domain is a seven-stranded β sheet. The small domain has either a three or four-stranded β sheet that is surrounded by α helices on one side. The cofactor PLP is covalently attached to a lysine residue in the large domain and is anchored in a way that allows the aromatic ring of PLP to pack against neighboring β strands. The active site is located in a cleft between the two domains at the interface between the two subunits. '''''Thus, enzymes of fold-type I have residues from both domains and both subunits involved in PLP-binding.''''' | ||
---- | ---- | ||
[[image:dopadecarb.png|thumb|200px|'''DOPA Decarboxylase''']][[image:super1.png|thumb|200px|'''DOPA decarboxylase superimposed on aspartate aminotransferase''']] | [[image:dopadecarb.png|thumb|200px|'''DOPA Decarboxylase''']][[image:super1.png|thumb|200px|'''DOPA decarboxylase superimposed on aspartate aminotransferase''']] | ||
| + | {{Clear}} | ||
---- | ---- | ||
===DOPA Decarboxylase=== | ===DOPA Decarboxylase=== | ||
| Line 48: | Line 51: | ||
---- | ---- | ||
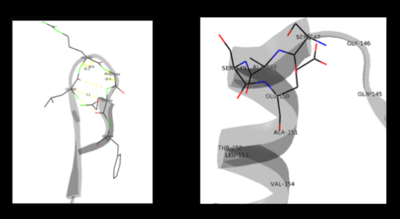
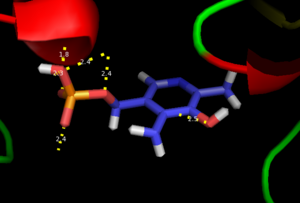
As well, a '''salt bridge''' exists between the carboxyl group of <scene name='DOPA_decarboxylase/Aspartic/1'>Asp271</scene> and the protonated pyridine nitrogen of PLP to further stabilize intermediate. Essentially, a salt bridge combines hydrogen bonding and electrostatic interactions (two common types non-covalent interactions). This interaction serves to provide an electron sink that can stabilize the carbanionic intermediates <ref name="jansonius">PMID:9914259 </ref> . PLP is further anchored to the protein by an extended '''hydrogen bond network''', as shown below. | As well, a '''salt bridge''' exists between the carboxyl group of <scene name='DOPA_decarboxylase/Aspartic/1'>Asp271</scene> and the protonated pyridine nitrogen of PLP to further stabilize intermediate. Essentially, a salt bridge combines hydrogen bonding and electrostatic interactions (two common types non-covalent interactions). This interaction serves to provide an electron sink that can stabilize the carbanionic intermediates <ref name="jansonius">PMID:9914259 </ref> . PLP is further anchored to the protein by an extended '''hydrogen bond network''', as shown below. | ||
| - | [[image:h-bonding.png|thumb|center|300px|'''H-bonding network of PLP in the active site''']] | + | [[image:h-bonding.png|thumb|center|300px|'''H-bonding network of PLP in the active site''']] |
| + | {{Clear}} | ||
---- | ---- | ||
The only two active site residues from the adjacent monomer, Ile-101 and Phe-103, are part of the substrate binding pocket. | The only two active site residues from the adjacent monomer, Ile-101 and Phe-103, are part of the substrate binding pocket. | ||
| Line 54: | Line 58: | ||
===Inhibitor Binding=== | ===Inhibitor Binding=== | ||
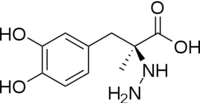
[[image:carbiDOPA.png|thumb|left|200px|'''carbiDOPA''']] | [[image:carbiDOPA.png|thumb|left|200px|'''carbiDOPA''']] | ||
| + | {{Clear}} | ||
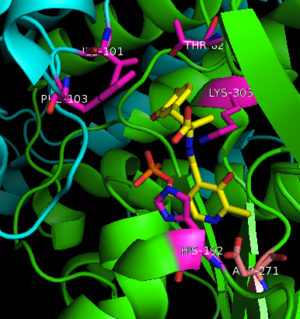
The inhibitor <scene name='DOPA_decarboxylase/Carbidopa/1'>carbiDOPA</scene> binds to the enzyme by forming a hydrazone linkage with PLP through its hydrazine moiety. The catechol ring of carbiDOPA is deeply buried in the active site cleft and is stabilized by <scene name='DOPA_decarboxylase/Vanderwaals/1'>van der waals contact</scene> with Ile-101 and Phe-103. The 4' hydroxyl group of the catechol ring participates in hydrogen bonding with <scene name='DOPA_decarboxylase/Thr-82/1'>Thr-82</scene>, further stabilizing the inhibitor in the active site cleft. PLP is further involved in substrate binding by forming a hydrogen bond to the 3' of the catechol ring. <scene name='DOPA_decarboxylase/His192/1'>His-192</scene>, a highly conserved residue of PLP-dependent decarboxylases <ref name="ishii">PMID:8889823 </ref> hydrogen bonds to the carboxylate group of carbiDOPA. | The inhibitor <scene name='DOPA_decarboxylase/Carbidopa/1'>carbiDOPA</scene> binds to the enzyme by forming a hydrazone linkage with PLP through its hydrazine moiety. The catechol ring of carbiDOPA is deeply buried in the active site cleft and is stabilized by <scene name='DOPA_decarboxylase/Vanderwaals/1'>van der waals contact</scene> with Ile-101 and Phe-103. The 4' hydroxyl group of the catechol ring participates in hydrogen bonding with <scene name='DOPA_decarboxylase/Thr-82/1'>Thr-82</scene>, further stabilizing the inhibitor in the active site cleft. PLP is further involved in substrate binding by forming a hydrogen bond to the 3' of the catechol ring. <scene name='DOPA_decarboxylase/His192/1'>His-192</scene>, a highly conserved residue of PLP-dependent decarboxylases <ref name="ishii">PMID:8889823 </ref> hydrogen bonds to the carboxylate group of carbiDOPA. | ||
[[image:actsite.png|thumb|center|300px|'''Key interactions between the active site residues, PLP, and carbiDOPA''']] | [[image:actsite.png|thumb|center|300px|'''Key interactions between the active site residues, PLP, and carbiDOPA''']] | ||
| - | + | {{Clear}} | |
'''Other inhibitors''' include [http://en.wikipedia.org/wiki/Benserazide Benserazide] (Serazide), α-Difluoromethyl-DOPA, and α-methyldopa. However, Benserazide is unapproved for use in the U.S. and is replaced by carbiDOPA instead. Both inhibitors act by preventing peripheral metabolism of L-DOPA, and cannot cross the blood-brain barrier. | '''Other inhibitors''' include [http://en.wikipedia.org/wiki/Benserazide Benserazide] (Serazide), α-Difluoromethyl-DOPA, and α-methyldopa. However, Benserazide is unapproved for use in the U.S. and is replaced by carbiDOPA instead. Both inhibitors act by preventing peripheral metabolism of L-DOPA, and cannot cross the blood-brain barrier. | ||
| Line 68: | Line 73: | ||
{{Clear}} | {{Clear}} | ||
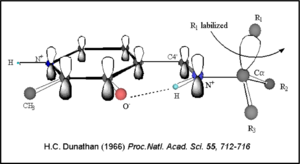
Once again, the transimination step (conversion of internal to external aldimine) is common to all PLP-dependent enzymes. The unique absorption wavelengths of the PLP intermediates has allowed for their straightforward detection (for example, an internal Schiff base in the enolimine form absorbs at 310-330 nm, whereas the quinonoid intermediate absorbs at ~500 nm). The orientation of the quinonoid intermediate allows for stereospecific decarboxylation of the substrate at the alpha carbon, as predicted by '''Dunathan's stereoelectronic hypothesis''' <ref name="dunathan">PMID:224217 </ref>, in which he proposed that the substrate binds PLP such that the external aldimine intermediate is oriented perpendicular to the coenzyme pi bonding system. In doing so, the sigma-pi orbital overlap in the transition state is maximized, thus maximizing the rate of the reaction. | Once again, the transimination step (conversion of internal to external aldimine) is common to all PLP-dependent enzymes. The unique absorption wavelengths of the PLP intermediates has allowed for their straightforward detection (for example, an internal Schiff base in the enolimine form absorbs at 310-330 nm, whereas the quinonoid intermediate absorbs at ~500 nm). The orientation of the quinonoid intermediate allows for stereospecific decarboxylation of the substrate at the alpha carbon, as predicted by '''Dunathan's stereoelectronic hypothesis''' <ref name="dunathan">PMID:224217 </ref>, in which he proposed that the substrate binds PLP such that the external aldimine intermediate is oriented perpendicular to the coenzyme pi bonding system. In doing so, the sigma-pi orbital overlap in the transition state is maximized, thus maximizing the rate of the reaction. | ||
| - | [[image:Dunathan.png|thumb|left|300px|'''Dunathan's Stereoeletronic Hypothesis, 1966''']] | + | [[image:Dunathan.png|thumb|left|300px|'''Dunathan's Stereoeletronic Hypothesis, 1966''']] |
| + | {{Clear}} | ||
This way, the developing p orbital is aligned for maximal overlap with the extended p system, lowering the energy of the transition state and increasing the rate of the reaction. As well, by controlling substrate orientation, the enzyme can distinguish between '''deprotonation''' and '''decarboxylation'''. | This way, the developing p orbital is aligned for maximal overlap with the extended p system, lowering the energy of the transition state and increasing the rate of the reaction. As well, by controlling substrate orientation, the enzyme can distinguish between '''deprotonation''' and '''decarboxylation'''. | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
===Mechanism Breakdown=== | ===Mechanism Breakdown=== | ||
| Line 96: | Line 97: | ||
With it's possible relation to degenerative dopamine-producing cells in the brain, administration of L-DOPA can increase the amount of synthesized dopamine in the nerve cell; whereas, direct treatment with dopamine is not sufficient as dopamine itself cannot pass the blood-brain barrier<ref name=Burkhard>PMID: 11685243 </ref>. Even still, only a small percentage of the dose actually reaches the nervous system, with the remaining majority being rapidly decarboxylated to dopamine in the blood stream. This dopamine-rich blood causes side effects of nausea, daytime sleepiness, orthostatic hypotension, involuntary movements, decreased appetite, insomnia, and cramping. Addition of a DDC inhibitor would block peripheral conversion to dopamine and allow a greater percentage of L-DOPA to reach the brain, causing an increase in brain dopamine levels, and reducing the side effects of dopamine-rich blood. | With it's possible relation to degenerative dopamine-producing cells in the brain, administration of L-DOPA can increase the amount of synthesized dopamine in the nerve cell; whereas, direct treatment with dopamine is not sufficient as dopamine itself cannot pass the blood-brain barrier<ref name=Burkhard>PMID: 11685243 </ref>. Even still, only a small percentage of the dose actually reaches the nervous system, with the remaining majority being rapidly decarboxylated to dopamine in the blood stream. This dopamine-rich blood causes side effects of nausea, daytime sleepiness, orthostatic hypotension, involuntary movements, decreased appetite, insomnia, and cramping. Addition of a DDC inhibitor would block peripheral conversion to dopamine and allow a greater percentage of L-DOPA to reach the brain, causing an increase in brain dopamine levels, and reducing the side effects of dopamine-rich blood. | ||
| - | |||
| - | |||
==Classification== | ==Classification== | ||
---- | ---- | ||
| Line 122: | Line 121: | ||
#'''Topology''': dopa decarboxylase | #'''Topology''': dopa decarboxylase | ||
| - | + | ||
==3D structures of DOPA decarboxylase== | ==3D structures of DOPA decarboxylase== | ||
| Line 128: | Line 127: | ||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | [[3rbf]], [[3rbl]] – hDDC – human<br /> | ||
| + | [[3rch]], [[8or9]], [[8ora]] – hDDC + PLP <br /> | ||
[[3k40]] – DDC – ''Drosophila melanogaster''<br /> | [[3k40]] – DDC – ''Drosophila melanogaster''<br /> | ||
[[1js3]] – pDDC + inhibitor – pig<br /> | [[1js3]] – pDDC + inhibitor – pig<br /> | ||
[[1js6]] - pDDC<br /> | [[1js6]] - pDDC<br /> | ||
| - | [[ | + | [[6eew]] – MpDDC + Trp – Madagascar periwinkle<br /> |
| - | [[ | + | [[6eem]] – MpDDC + Tyr<br /> |
| + | [[6liu]] – poDDC - poppy<br /> | ||
| + | [[6liv]] – poDDC + PLP derivative<br /> | ||
| + | [[8x0p]] – poDDC + PLP + carbidopa | ||
| + | [[8x0o]] – poDDC + Tyr + PLP derivative | ||
| + | [[7xin]] – DDC + PLP - ''Pseudomonas'' | ||
==References== | ==References== | ||
| Line 138: | Line 144: | ||
<references /> | <references /> | ||
| - | + | </StructureSection> | |
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Brittany Todd, Michal Harel, David Canner, Alexander Berchansky, Brian Hernandez