Sandbox Reserved 819
From Proteopedia
(Difference between revisions)
| (10 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
{{Sandbox_Reserved_ESBS}} | {{Sandbox_Reserved_ESBS}} | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| + | |||
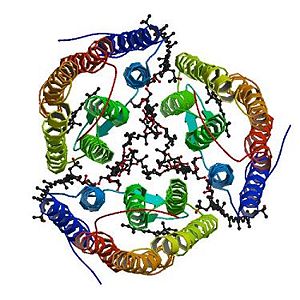
<StructureSection load='2z55' size='300' side='right' caption='2z55: cristal made of four Archaerhodopsin-2' scene=''> | <StructureSection load='2z55' size='300' side='right' caption='2z55: cristal made of four Archaerhodopsin-2' scene=''> | ||
| + | =2Z55= | ||
| + | __TOC__ | ||
==Introduction== | ==Introduction== | ||
| Line 56: | Line 59: | ||
Furthermore, the bacterioruberin is essential because it plays a structural role for the trimerization of aR2: it mediates interactions between neighbouring monomers. | Furthermore, the bacterioruberin is essential because it plays a structural role for the trimerization of aR2: it mediates interactions between neighbouring monomers. | ||
| - | When the bacterioruberin is bound to the Archaerhodopsin-2, its polyene chain is between the A and B helices of one monomere and the D and E helices of an adjacent one. One end of the | + | When the bacterioruberin is bound to the Archaerhodopsin-2, its polyene chain is between the A and B helices of one monomere and the D and E helices of an adjacent one. One end of the bacterioruberin is next to the cytoplasmic membrane surfaces and thus is able to interact with the hydrophilic residus of two monomers. The other end of the bacterioruberin protrudes out of the extracellular membrane. <ref name="multiple">PMID:18082767</ref> |
More precisely, it binds to: the B chain thanks to a hydrogen bond with the <scene name='56/568017/Threonine_112/1'>Threonine 112</scene>, the <scene name='56/568017/Tyrosine/1'>Tyrosine 156</scene> and thanks to an electrostatic bond with the HOH 304; the D chain thanks to a hydrogen bond with the Tyrosine 156; the E chain thanks to a hydrogen bond with the Tyrosine 156. Others bonds exist like van-der-waals bonds [http://www.ebi.ac.uk/pdbe-site/pdbemotif/?tab=boundmolecule&pdb=2z55&ligandCode3letter=22B]. | More precisely, it binds to: the B chain thanks to a hydrogen bond with the <scene name='56/568017/Threonine_112/1'>Threonine 112</scene>, the <scene name='56/568017/Tyrosine/1'>Tyrosine 156</scene> and thanks to an electrostatic bond with the HOH 304; the D chain thanks to a hydrogen bond with the Tyrosine 156; the E chain thanks to a hydrogen bond with the Tyrosine 156. Others bonds exist like van-der-waals bonds [http://www.ebi.ac.uk/pdbe-site/pdbemotif/?tab=boundmolecule&pdb=2z55&ligandCode3letter=22B]. | ||
| Line 73: | Line 76: | ||
The 2,3-di-phytanyl-glycerol [http://www.ebi.ac.uk/pdbe-srv/pdbechem/chemicalCompound/show/L2P] (C43 H88 O3) is an archaeol (di-O-phytanylglycerol). This is a double ether of sn-1-glycerol where positions 2 and 3 are bound to phytanyl residues. The archaeols are Archaea homologs of diacylglycerols (DAGs). | The 2,3-di-phytanyl-glycerol [http://www.ebi.ac.uk/pdbe-srv/pdbechem/chemicalCompound/show/L2P] (C43 H88 O3) is an archaeol (di-O-phytanylglycerol). This is a double ether of sn-1-glycerol where positions 2 and 3 are bound to phytanyl residues. The archaeols are Archaea homologs of diacylglycerols (DAGs). | ||
| - | It interacts with the aR2 surface and the carbohydrate <scene name='56/568017/Glc/1'>GLC</scene>. It binds to: the A chain thanks to a covalent bond with the carbohydrate alpha-D-glucose 281 (GLC) and thanks to a hydrogen bond with the <scene name='56/568017/ | + | It interacts with the aR2 surface and the carbohydrate <scene name='56/568017/Glc/1'>GLC</scene>. It binds to: the A chain thanks to a covalent bond with the carbohydrate alpha-D-glucose 281 (GLC) and thanks to a hydrogen bond with the <scene name='56/568017/Tyr85/1'>Tyrosine 85</scene>; the B chain thanks to a covalent bond with the carbohydrate alpha-D-glucose 281 (GLC) and thanks to a hydrogen bond with the Tyrosine 85; the D chain thanks to a covalent bond with the carbohydrate alpha-D-glucose 281 (GLC) and thanks to a hydrogen bond with the Tyrosine 85; the E chain thanks to a covalent bond with the carbohydrate alpha-D-glucose 284 (GLC) and thanks to a hydrogen bond with the Tyrosine 85. Others bonds exist like van-der-waals bonds [http://www.ebi.ac.uk/pdbe-site/pdbemotif/?tab=boundmolecule&pdb=2z55&ligandCode3letter=L2P]. |
| Line 89: | Line 92: | ||
56% of the Archaeorhodopsin-2 sequence is identical to the [[Bacteriorhodopsin]] sequence.<ref name="seq">PMID: 1654776</ref> | 56% of the Archaeorhodopsin-2 sequence is identical to the [[Bacteriorhodopsin]] sequence.<ref name="seq">PMID: 1654776</ref> | ||
| - | Most amino acids that play a role in the trimerization are not conserved between the two proteins. For instance, the counterparts of some hydrophobic residues of the Archaerhodopsin-2 (the one interacting with the polyene chain of the bacterioruberin) have a different volume. Another difference is the fact that the | + | Most amino acids that play a role in the trimerization are not conserved between the two proteins. For instance, the counterparts of some hydrophobic residues of the Archaerhodopsin-2 (the one interacting with the polyene chain of the bacterioruberin) have a different volume. Another difference is the fact that the polar residues of the Archaerhodopsin-2 (responsible for the hydrogen bonds with the bacterioruberin) are replaced in the Bacteriorhodopsin by hydrophobic amino acids. |
| - | However the global structures of Archaeorhodopsin-2 and | + | However the global structures of Archaeorhodopsin-2 and Bacteriorhodopsin are really similar, especially at the level of the open space between the monomers. The interaction between the monomers of the Bacteriorhodopsin is also mediated by lipids: diphytanyl diether phospholipids instead of Bacterioruberin. |
This similarity of structure forms the basis of several hypothesis concerning the mechanisms of the Archaeorhodopsin-2 <ref name="multiple">PMID:18082767</ref> | This similarity of structure forms the basis of several hypothesis concerning the mechanisms of the Archaeorhodopsin-2 <ref name="multiple">PMID:18082767</ref> | ||
Current revision
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |
| |||||||||||
3D structures of Archaerhodopsin-2 and Bacteriorhodopsin
2ei4-Trimeric structure of Archaerhodopsin-2
1vgo-Crystal Structure of Archaerhodopsin-2
1uaz-Crystal structure of archaerhodopsin-1
1iw6-Crystal Structure of the Ground State of Bacteriorhodopsin
References
- ↑ 1.0 1.1 Uegaki K, Sugiyama Y, Mukohata Y. Archaerhodopsin-2, from Halobacterium sp. aus-2 further reveals essential amino acid residues for light-driven proton pumps. Arch Biochem Biophys. 1991 Apr;286(1):107-10. PMID:1654776
- ↑ 2.0 2.1 2.2 2.3 Yoshimura K, Kouyama T. Structural role of bacterioruberin in the trimeric structure of archaerhodopsin-2. J Mol Biol. 2008 Feb 1;375(5):1267-81. Epub 2007 Nov 22. PMID:18082767 doi:10.1016/j.jmb.2007.11.039
- ↑ Shammohammadi, H.R., Protective roles of bacterioruberin and intracellular KCl in the resistance of Halobacterium salinarium against DNA-damaging agents, J Radiat Res, 1998, 39(4):251.
- ↑ Ide, H., Takeshi, S., Hiroaki, T., Studies on the antioxidation activity of bacterioruberin, Urakami Found Mem, 1998, 6:127–33.
Proteopedia page contributors and editors
Lydwine Germain, Allan Bernard