This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 814
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 7: | Line 7: | ||
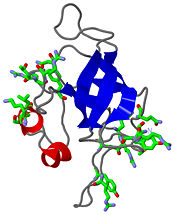
== Structure == | == Structure == | ||
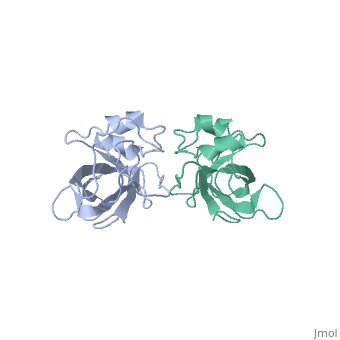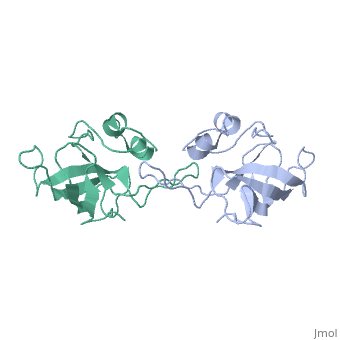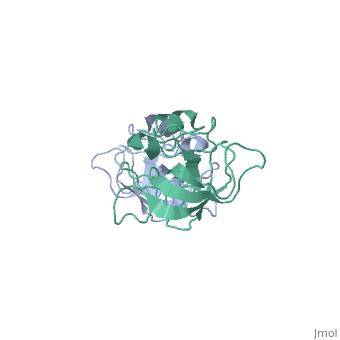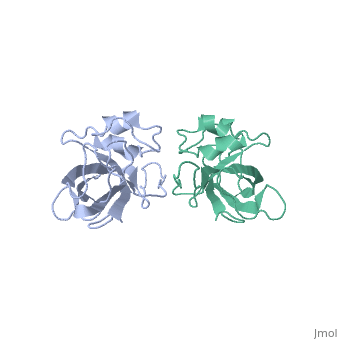
| - | The L14 subunit has a molecular mass of 13.3 kDa and contains 122 amino acids. The molecule is extremly compact so water molecule are exclude. It belong to α+β class of proteins. It is composed of five stranded <scene name='56/568012/Barrel/1'>beta barrel</scene> : β1-β2-β3-β4-β7 all antiparallel, stabilized by hydrogen-bounding, a C-terminal region which contains the two small <scene name='56/568012/Helix/2'>alpha-helixes</scene> and a beta-ribbon. The beta-barrel contains a hydrophobic core of highly conserved residues : Ala, Leu, Ile, Val, Cys. The beta-barrel is stabilized by some hydrogen-bonding such as a bonding between loop 2 and loops 4 and 8. <scene name='56/568012/Loopstab/1'>Loop 4 interact with the Ser14 of the loop 2 by the residue 51</scene>. | + | The L14 subunit has a molecular mass of 13.3 kDa and contains 122 amino acids. The molecule is extremly compact so water molecule are exclude. It belong to α+β class of proteins. It is composed of five stranded <scene name='56/568012/Barrel/1'>beta barrel</scene> : β1-β2-β3-β4-β7 all antiparallel, stabilized by hydrogen-bounding, a C-terminal region which contains the two small <scene name='56/568012/Helix/2'>alpha-helixes</scene> and a beta-ribbon : the extended loop8. The beta-barrel contains a hydrophobic core of highly conserved residues : Ala, Leu, Ile, Val, Cys. The beta-barrel is stabilized by some hydrogen-bonding such as a bonding between loop 2 and loops 4 and 8. <scene name='56/568012/Loopstab/1'>Loop 4 interact with the Ser14 of the loop 2 by the residue 51</scene>. The loops 3 which connects β2 and β3 allows to close this end with the hydrophobic side chains. The top of the β-barrel is made with 2 valines 51 and 54 in loop 4. |
| + | The loop 2 has an unusual and highly structure turn which contains the most conserved sequence, stabilized by a hydrogen-bonding complex: the alide protons and carbonyl oxygène of 3 residues and a water molecule. The turn allows to stabilize loop 4 and loop 8. Loop8 has several interactions. Finally, three structural waters (206–208) are involved in the stabilization of the local conformation around residue 91 in loop8 and the N terminus of helix α2. | ||
== Bidding sites == | == Bidding sites == | ||
| Line 25: | Line 26: | ||
Christopher Davies, Stephen W White, V Ramakrishnan. The crystal structure of ribosomal protein L14 reveals an important organizational component of the translational apparatus. | Christopher Davies, Stephen W White, V Ramakrishnan. The crystal structure of ribosomal protein L14 reveals an important organizational component of the translational apparatus. | ||
| + | |||
| + | L. Mattheakis, L. Vu, F. Sor, M. Nomura ; Octobre 1988. Retroregulation of the synthesis of ribosomal protein L14 and L24 by feedback repressor S8 in Escherichia coli.. | ||
By Bonhomme Clémence and Dilda Nathan</StructureSection><!-- PLEASE DO NOT DELETE THIS TEMPLATE --> | By Bonhomme Clémence and Dilda Nathan</StructureSection><!-- PLEASE DO NOT DELETE THIS TEMPLATE --> | ||
{{Sandbox_Reserved_ESBS}} | {{Sandbox_Reserved_ESBS}} | ||
<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | <!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
Current revision
RIBOSOMAL PROTEIN L14
| |||||||||||
| This Sandbox is Reserved from 06/12/2018, through 30/06/2019 for use in the course "Structural Biology" taught by Bruno Kieffer at the University of Strasbourg, ESBS. This reservation includes Sandbox Reserved 1480 through Sandbox Reserved 1543. |
To get started:
More help: Help:Editing |