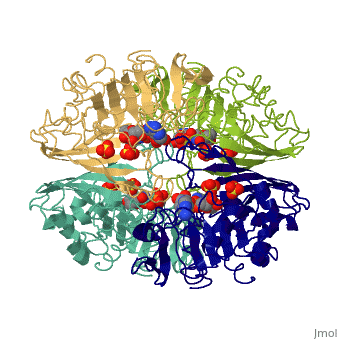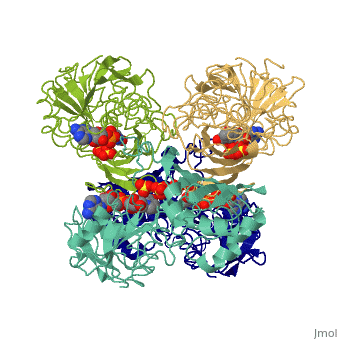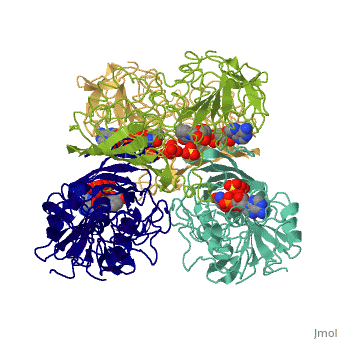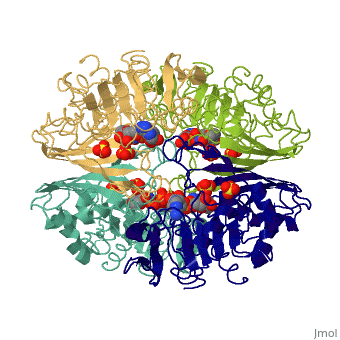
We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
3gpd
From Proteopedia
(Difference between revisions)
| (16 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | [[Image:3gpd.gif|left|200px]]<br /><applet load="3gpd" size="350" color="white" frame="true" align="right" spinBox="true" | ||
| - | caption="3gpd, resolution 3.5Å" /> | ||
| - | '''TWINNING IN CRYSTALS OF HUMAN SKELETAL MUSCLE D-GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE'''<br /> | ||
| - | == | + | ==TWINNING IN CRYSTALS OF HUMAN SKELETAL MUSCLE D-GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE== |
| - | + | <StructureSection load='3gpd' size='340' side='right'caption='[[3gpd]], [[Resolution|resolution]] 3.50Å' scene=''> | |
| + | == Structural highlights == | ||
| + | <table><tr><td colspan='2'>[[3gpd]] is a 2 chain structure with sequence from [https://en.wikipedia.org/wiki/Homo_sapiens Homo sapiens]. The February 2004 RCSB PDB [https://pdb.rcsb.org/pdb/static.do?p=education_discussion/molecule_of_the_month/index.html Molecule of the Month] feature on ''The Glycolytic Enzymes'' by David S. Goodsell is [https://dx.doi.org/10.2210/rcsb_pdb/mom_2004_2 10.2210/rcsb_pdb/mom_2004_2]. Full crystallographic information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=3GPD OCA]. For a <b>guided tour on the structure components</b> use [https://proteopedia.org/fgij/fg.htm?mol=3GPD FirstGlance]. <br> | ||
| + | </td></tr><tr id='method'><td class="sblockLbl"><b>[[Empirical_models|Method:]]</b></td><td class="sblockDat" id="methodDat">X-ray diffraction, [[Resolution|Resolution]] 3.5Å</td></tr> | ||
| + | <tr id='ligand'><td class="sblockLbl"><b>[[Ligand|Ligands:]]</b></td><td class="sblockDat" id="ligandDat"><scene name='pdbligand=NAD:NICOTINAMIDE-ADENINE-DINUCLEOTIDE'>NAD</scene>, <scene name='pdbligand=SO4:SULFATE+ION'>SO4</scene></td></tr> | ||
| + | <tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[https://proteopedia.org/fgij/fg.htm?mol=3gpd FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=3gpd OCA], [https://pdbe.org/3gpd PDBe], [https://www.rcsb.org/pdb/explore.do?structureId=3gpd RCSB], [https://www.ebi.ac.uk/pdbsum/3gpd PDBsum], [https://prosat.h-its.org/prosat/prosatexe?pdbcode=3gpd ProSAT]</span></td></tr> | ||
| + | </table> | ||
| + | == Function == | ||
| + | [https://www.uniprot.org/uniprot/G3P_HUMAN G3P_HUMAN] Has both glyceraldehyde-3-phosphate dehydrogenase and nitrosylase activities, thereby playing a role in glycolysis and nuclear functions, respectively. Participates in nuclear events including transcription, RNA transport, DNA replication and apoptosis. Nuclear functions are probably due to the nitrosylase activity that mediates cysteine S-nitrosylation of nuclear target proteins such as SIRT1, HDAC2 and PRKDC. Modulates the organization and assembly of the cytoskeleton. Facilitates the CHP1-dependent microtubule and membrane associations through its ability to stimulate the binding of CHP1 to microtubules (By similarity). Glyceraldehyde-3-phosphate dehydrogenase is a key enzyme in glycolysis that catalyzes the first step of the pathway by converting D-glyceraldehyde 3-phosphate (G3P) into 3-phospho-D-glyceroyl phosphate. Component of the GAIT (gamma interferon-activated inhibitor of translation) complex which mediates interferon-gamma-induced transcript-selective translation inhibition in inflammation processes. Upon interferon-gamma treatment assembles into the GAIT complex which binds to stem loop-containing GAIT elements in the 3'-UTR of diverse inflammatory mRNAs (such as ceruplasmin) and suppresses their translation.<ref>PMID:3170585</ref> <ref>PMID:11724794</ref> <ref>PMID:23071094</ref> | ||
| + | == Evolutionary Conservation == | ||
| + | [[Image:Consurf_key_small.gif|200px|right]] | ||
| + | Check<jmol> | ||
| + | <jmolCheckbox> | ||
| + | <scriptWhenChecked>; select protein; define ~consurf_to_do selected; consurf_initial_scene = true; script "/wiki/ConSurf/gp/3gpd_consurf.spt"</scriptWhenChecked> | ||
| + | <scriptWhenUnchecked>script /wiki/extensions/Proteopedia/spt/initialview03.spt</scriptWhenUnchecked> | ||
| + | <text>to colour the structure by Evolutionary Conservation</text> | ||
| + | </jmolCheckbox> | ||
| + | </jmol>, as determined by [http://consurfdb.tau.ac.il/ ConSurfDB]. You may read the [[Conservation%2C_Evolutionary|explanation]] of the method and the full data available from [http://bental.tau.ac.il/new_ConSurfDB/main_output.php?pdb_ID=3gpd ConSurf]. | ||
| + | <div style="clear:both"></div> | ||
| - | == | + | ==See Also== |
| - | + | *[[Aldehyde dehydrogenase 3D structures|Aldehyde dehydrogenase 3D structures]] | |
| - | [[ | + | *[[Glyceraldehyde-3-Phosphate Dehydrogenase|Glyceraldehyde-3-Phosphate Dehydrogenase]] |
| + | *[[Glyceraldehyde-3-phosphate dehydrogenase 3D structures|Glyceraldehyde-3-phosphate dehydrogenase 3D structures]] | ||
| + | == References == | ||
| + | <references/> | ||
| + | __TOC__ | ||
| + | </StructureSection> | ||
[[Category: Homo sapiens]] | [[Category: Homo sapiens]] | ||
| - | [[Category: | + | [[Category: Large Structures]] |
| + | [[Category: RCSB PDB Molecule of the Month]] | ||
[[Category: The Glycolytic Enzymes]] | [[Category: The Glycolytic Enzymes]] | ||
| - | [[Category: Campbell | + | [[Category: Campbell JC]] |
| - | [[Category: Watson | + | [[Category: Watson HC]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
TWINNING IN CRYSTALS OF HUMAN SKELETAL MUSCLE D-GLYCERALDEHYDE-3-PHOSPHATE DEHYDROGENASE
| |||||||||||