We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Proteinase
From Proteopedia
(Difference between revisions)
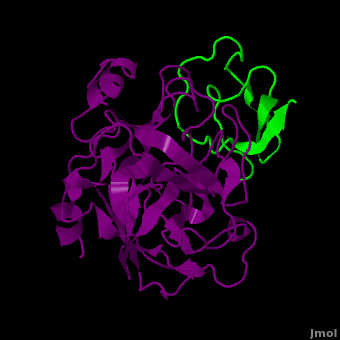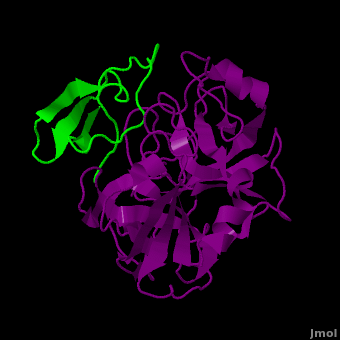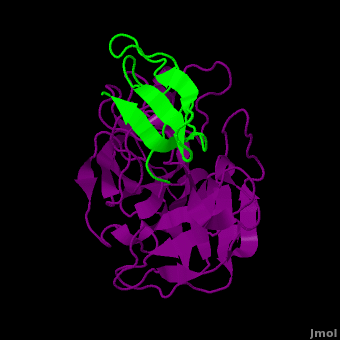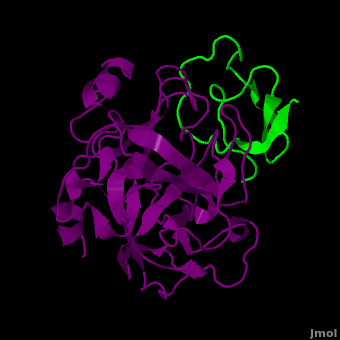
(New page: <StructureSection load='2duj' size='340' side='right' caption='E. coli proteinase K complex with lactoferrin peptide and Ca+2 (PDB code 2duj)' scene=''> '''Proteinase''' (PRO) are enz...) |
|||
| (30 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='' size='350' side='right' scene='Journal:JBSD:39/Cv/11' caption=''> | ||
| + | __TOC__ | ||
| + | ==Function== | ||
| - | + | '''Proteinase''' (PRO) are enzymes which hydrolyze peptide bonds. They are classified by the amino acid site of their cleavage or by the pH at which they are active.<br /> | |
| - | '''Proteinase''' (PRO) are enzymes which hydrolyze peptide bonds. They are classified by the amino acid site of their cleavage or by the pH at which they are active. PRO B is a serine protease. For more details | + | * '''PRO B''' is a serine protease<ref>PMID:3325823</ref>. For more details see [[Streptomyces griseus proteinase B]].<br /> |
| + | * '''PRO A''' is a carboxylproteinase<ref>PMID:6799292</ref>.<br /> | ||
| + | * '''PRO K''' is a serine protease which cleaves proteins preferentially after hydrophobic residues<ref>PMID:9606141</ref>. Calcium ions contribute to the stability of the enzyme. PRO K is active over a wide pH range and is used in molecular biology to inactivate nucleases from preparations of DNA or RNA. PRO K is used in the partial proteolysis of lactoferrin into its N- and C-lobe. The two lobes of lactoferrin have different antimicrobial and antifungal properties. PRO K can digest hair (keratin).<br /> | ||
| + | *'''Endothiapepsin''' is an '''aspartic PRO''' from ''Cryphonectria parasitica''<ref>PMID:1525155</ref>.<br /> | ||
| + | *'''Saccharopepsin''' is an '''aspartic PRO''' from yeast<ref>PMID:17447722</ref>.<br /> | ||
| + | *'''Falcipain''' is an '''cystein PRO''' from ''Plasmodium falciparum''<ref>PMID:21660657</ref>.<br /> | ||
| + | For '''cysteine PRO''' from ''Trypanosoma cruzi'' see [[Cruzain]]. | ||
==3D structures of proteinase== | ==3D structures of proteinase== | ||
| + | [[Proteinase 3D structures]] | ||
| - | + | </StructureSection> | |
| - | '''PRO A''' | ||
| - | |||
| - | 2sga – SgPRO – ''Streptomyces griseus'' | ||
| - | 2jxr, 1fmu, 1fmx – yPRO - yeast | ||
| - | 1sgc - SgPRO + chymostatin A | ||
| - | 3sga, 4sga, 5sga - SgPRO + polypeptide inhibitor | ||
| - | 1dp5, 1dpj, 1g0v - yPRO + polypeptide inhibitor IA3 | ||
| - | 1fq5 - yPRO + inhibitor | ||
| - | |||
| - | '''PRO B''' | ||
| - | |||
| - | 3sgb – SgPRO + turkey ovomucoid inhibitor | ||
| - | 1sgp, 1sgq, 1sgr, 1cso, 1ct0, 1ct2, 1ct4, 1ds2, 2sgp, 2nu3, 2nu4 – SgPRO + turkey ovomucoid inhibitor (mutant) | ||
| - | 4sgb - SgPRO + potato inhibitor | ||
| - | |||
| - | '''PRO K''' | ||
| - | |||
| - | [[2prk]], [[1cnm]], [[1egq]], [[2id8]], [[2g4v]], [[2v8b]], [[3gt3]], [[3gt4]], [[3d9q]], [[3ddz]], [[3de0]] , [[3de1]], [[3de2]], [[3de3]], [[3de4]], [[3de5]], [[3de6]], [[3de7]], [[3dvq]], [[3dvr]], [[3dvs]], [[3dw1]], [[3dw3]], [[3dwe]], [[3i2y]], [[3i30]], [[3i37]], [[3i34]], [[3l1k]], [[3aj8]], [[3aj9]], [[3q40]], [[3q5g]], [[3qmp]], [[4b5l]], [[4fon]] – EaPRO + Ca – ''Engyodontium album'' <br /> | ||
| - | [[1ic6]] – EaPRO (mutant) + Ca <br /> | ||
| - | [[1ptk]], [[1ht3]] – EaPRO + Ca + Hg <br /> | ||
| - | [[2pkc]] – EaPRO + Na <br /> | ||
| - | [[4dj5]] – EaPRO <br /> | ||
| - | |||
| - | ''PRO K complex with peptide'' | ||
| - | |||
| - | [[3prk]], [[1p7v]], [[1p7w]] – EaPRO + Ca + peptide inhibitor <br /> | ||
| - | [[1bjr]], [[2dqk]], [[2duj]] – EaPRO + Ca + lactoferrin peptide <br /> | ||
| - | [[2hd4]] – EaPRO + Ca + lactoferrin peptide inhibitor<br /> | ||
| - | [[2dp4]], [[3ptl]] – EaPRO + lactoferrin peptide <br /> | ||
| - | [[1pek]], [[1pfg]] – EaPRO + peptide inhibitor <br /> | ||
| - | [[1pj8]] – EaPRO + Hg + substrate analog peptide <br /> | ||
| - | [[2hpz]], [[2pq2]] – EaPRO + Ca + peptide <br /> | ||
| - | [[3osz]] – EaPRO + Ca + antimicrobial peptide <br /> | ||
| - | [[2b6n]] – PRO + tripeptide - ''Serratia''<br /> | ||
| - | |||
| - | ''PRO K complex with small molecule'' | ||
| - | |||
| - | [[2pwb]] – EaPRO + Ca + coumarin <br /> | ||
| - | [[2pyz]] – EaPRO + Ca + auramine <br /> | ||
| - | [[2pwa]] – EaPRO + Ca + alanine boronic acid <br /> | ||
| - | [[1oyo]] – EaPRO + Ca + melanin monomer <br /> | ||
| - | [[3dyb]] – EaPRO + Ca + digalacturonic acid <br /> | ||
| - | |||
| - | |||
| - | '''PRO 3C''' | ||
| - | |||
| - | 1qa7 – PRO – Hepatitis virus | ||
| - | 2vb0 - PRO – Coxsakievirus | ||
| - | |||
| - | '''H2-PRO''' | ||
| - | |||
| - | 1wni – PRO – ''Trimeresurus flavoviridis'' | ||
| - | |||
| - | '''Aspartic PRO''' | ||
| - | |||
| - | 2asi – PRO – ''Rhizomucor miehei'' | ||
| - | 1zap – CaPRO – ''Candida albicans'' | ||
| - | 1izd - AoPRO – ''Aspergillus oryzae'' | ||
| - | 1eag – CaPRO + inhibitor | ||
| - | 1fq4 - yPRO + inhibitor | ||
| - | 1j71 - PRO + polypeptide inhibitor – ''Candida tropicalis'' | ||
| - | 1ize - AoPRO + polypeptide-statine inhibitor | ||
| - | 1wkr - PRO + polypeptide-statine inhibitor – ''Irpex lacteus'' | ||
| - | |||
| - | '''Cysteine PRO''' | ||
| - | |||
| - | 2hrv – PRO 2A – human rhinovirus | ||
| - | |||
| - | '''Serine PRO''' | ||
| - | |||
| - | 1s2n, 1sh7 – PRO – Vibrio | ||
| - | 3s9a, 3s9b – RvPRO – Siamese Russell’s viper | ||
| - | 3s9c, 3sbk – RvPRO + human factor V polypeptide | ||
| - | 1ga1, 1ga4, 1ga6, 1nlu – PsPRO + iodotyrostatin fragment – ''Pseudomonas'' | ||
| - | 1kdv, 1kdy, 1kdz, 1ke1, 1ke2 - PsPRO + polypeptide inhibitor | ||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | |||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
[[Category: Topic Page]] | [[Category: Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Moehle CM, Tizard R, Lemmon SK, Smart J, Jones EW. Protease B of the lysosomelike vacuole of the yeast Saccharomyces cerevisiae is homologous to the subtilisin family of serine proteases. Mol Cell Biol. 1987 Dec;7(12):4390-9. PMID:3325823
- ↑ Mechler B, Wolf DH. Analysis of proteinase A function in yeast. Eur J Biochem. 1981 Dec;121(1):47-52. PMID:6799292
- ↑ Petsch D, Deckwer WD, Anspach FB. Proteinase K digestion of proteins improves detection of bacterial endotoxins by the Limulus amebocyte lysate assay: application for endotoxin removal from cationic proteins. Anal Biochem. 1998 May 15;259(1):42-7. doi: 10.1006/abio.1998.2655. PMID:9606141 doi:http://dx.doi.org/10.1006/abio.1998.2655
- ↑ Cooper J, Quail W, Frazao C, Foundling SI, Blundell TL, Humblet C, Lunney EA, Lowther WT, Dunn BM. X-ray crystallographic analysis of inhibition of endothiapepsin by cyclohexyl renin inhibitors. Biochemistry. 1992 Sep 8;31(35):8142-50. PMID:1525155
- ↑ Parr CL, Keates RA, Bryksa BC, Ogawa M, Yada RY. The structure and function of Saccharomyces cerevisiae proteinase A. Yeast. 2007 Jun;24(6):467-80. doi: 10.1002/yea.1485. PMID:17447722 doi:http://dx.doi.org/10.1002/yea.1485
- ↑ Rosenthal PJ. Falcipains and other cysteine proteases of malaria parasites. Adv Exp Med Biol. 2011;712:30-48. doi: 10.1007/978-1-4419-8414-2_3. PMID:21660657 doi:http://dx.doi.org/10.1007/978-1-4419-8414-2_3
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, Alexander Berchansky, Karsten Theis