Sandbox Reserved 911
From Proteopedia
| (8 intermediate revisions not shown.) | |||
| Line 9: | Line 9: | ||
==Hydrolase Information== | ==Hydrolase Information== | ||
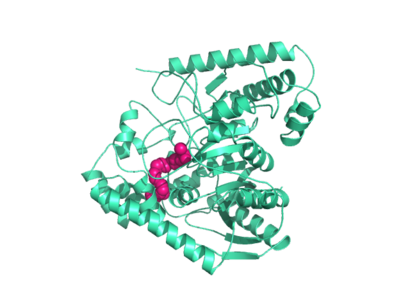
| - | Crystal structures of FAAH show that the enzyme is a <scene name='57/573125/2vya/9'>homodimer</scene> (monomers in different colors) in solution, with each subunit having a mass of 63 kD <ref name="1MT5"/>. The protein's <scene name='57/573125/2vya/11'>twisted Beta sheet core</scene> of 11 strands (blue) is surrounded by 24 <scene name='57/573125/2vya/12'>alpha helices</scene> (green). The enzyme is embedded in the cell membrane to catch the lipid signaling molecules that can diffuse through membranes; the hydrolase has a membrane binding cap, a <scene name='57/573125/2vya/14'>helix-turn-helix motif</scene> consisting of alpha helices 18 and 19. These helices present hydrophobic amino acid residues that help FAAH interact with the hydrophobic region of the lipid bilayer <ref name="1MT5"/>. Specifically, <scene name='57/573125/2vya/13'>alpha helix 18's hydrophobic residues</scene> (black) interact with the hydrophobic region of the membrane to anchor the enzyme in the lipid bilayer. The FAAH structure shows an entry channel leading from the lipid bilayer to the enzyme's active site, providing a path for endocannabinoids to enter the hydrolase. This entry channel is amphipathic to accommodate the entire lipid signaling molecule. Hydrophobic amino acid residues interact with the lipid signaling molecules' nonpolar tails, while charged R486 and D403 residues in the entry channel accommodate the polar head groups. In addition, FAAH possesses an [http://lem.ch.unito.it/didattica/infochimica/2008_Cioccolato/immagini/strutturafaah.jpg exit channel] leading from the active site to the cell's cytoplasm, allowing the release of polar compounds released from lipid cleavage and the entry of water molecules necessary for the FAAH mechanism to proceed <ref name="1MT5"/>. Different inhibitors have been designed to learn more about species selectivity <ref name="2VYA">PMID:18753625</ref> and binding flexibility <ref>PMID:19722626</ref> in FAAH. For example, the <scene name='57/573125/2vya/3'>PF-750 inhibitor</scene> (red) is the inhibitor used on a humanized rat FAAH protein. Although PF-750 showed preference for human FAAH, rat FAAH is easier to express; this demonstrates species selectivity of inhibitors <ref name="2VYA"/>. | + | Crystal structures of FAAH show that the enzyme is a <scene name='57/573125/2vya/9'>homodimer</scene> (monomers in different colors) in solution, with each subunit having a mass of 63 kD <ref name="1MT5"/>. The protein's <scene name='57/573125/2vya/11'>twisted Beta sheet core</scene> of 11 strands (blue) is surrounded by 24 <scene name='57/573125/2vya/12'>alpha helices</scene> (green). The enzyme is embedded in the cell membrane to catch the lipid signaling molecules that can diffuse through membranes; the hydrolase has a membrane binding cap, a <scene name='57/573125/2vya/14'>helix-turn-helix motif</scene> consisting of alpha helices 18 and 19. These helices present hydrophobic amino acid residues that help FAAH interact with the hydrophobic region of the lipid bilayer <ref name="1MT5"/>. Specifically, <scene name='57/573125/2vya/13'>alpha helix 18's hydrophobic residues</scene> (black) interact with the hydrophobic region of the membrane to anchor the enzyme in the lipid bilayer. The FAAH structure shows an entry channel leading from the lipid bilayer to the enzyme's active site, providing a path for endocannabinoids to enter the hydrolase. This <scene name='57/573125/2vya/16'>entry channel</scene> is amphipathic to accommodate the entire lipid signaling molecule. Hydrophobic amino acid residues (green) interact with the lipid signaling molecules' nonpolar tails, while charged R486 and D403 residues (black) in the entry channel accommodate the polar head groups. In addition, FAAH possesses an [http://lem.ch.unito.it/didattica/infochimica/2008_Cioccolato/immagini/strutturafaah.jpg exit channel] leading from the active site to the cell's cytoplasm, allowing the release of polar compounds released from lipid cleavage and the entry of water molecules necessary for the FAAH mechanism to proceed <ref name="1MT5"/>. Different inhibitors have been designed to learn more about species selectivity <ref name="2VYA">PMID:18753625</ref> and binding flexibility <ref>PMID:19722626</ref> in FAAH. For example, the <scene name='57/573125/2vya/3'>PF-750 inhibitor</scene> (red) is the inhibitor used on a humanized rat FAAH protein. Although PF-750 showed preference for human FAAH, rat FAAH is easier to express; this demonstrates species selectivity of inhibitors <ref name="2VYA"/>. |
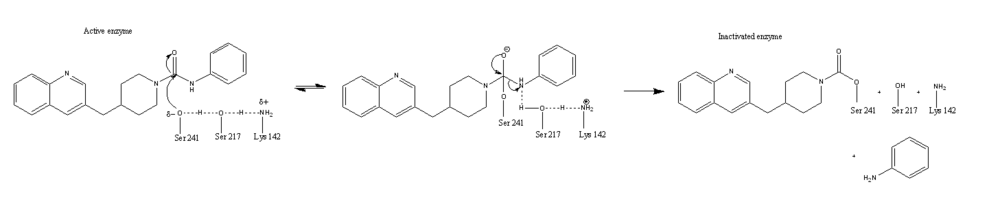
==Catalytic Triad== | ==Catalytic Triad== | ||
| Line 17: | Line 17: | ||
==Relationship to other proteins== | ==Relationship to other proteins== | ||
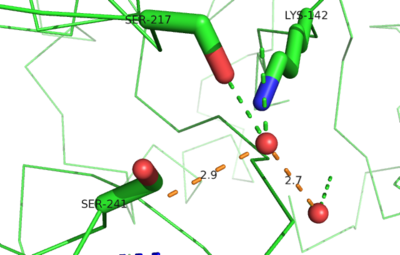
| - | The hydrolytic water molecules important to FAAH's function suggest an evolutionary relationship of this hydrolase to other enzymes. The structures of other [http://en.wikipedia.org/wiki/Serine_hydrolase serine hydrolases] also display a catalytic water molecule in their active sites. Because hydrolases that are non-homologous to FAAH also require a water molecule to cleave bonds, a functional convergance has inferred between amidase signature enzymes (such as FAAH) and other classes of serine proteases <ref name="3LJ6"/>. | + | The hydrolytic water molecules important to FAAH's function suggest an evolutionary relationship of this hydrolase to other enzymes. The structures of other [http://en.wikipedia.org/wiki/Serine_hydrolase serine hydrolases] also display a catalytic water molecule in their active sites. Because hydrolases that are non-homologous to FAAH also require a water molecule to cleave bonds, a functional convergance has inferred between amidase signature enzymes (such as FAAH) and other classes of [http://en.wikipedia.org/wiki/Serine_protease serine proteases] <ref name="3LJ6"/>. |
| - | [[Image: | + | [[Image:3LJ6_Image4.png|400 px|left|thumb|Figure3: FAAH catalytic site with water molecules bound; Protein in green, Water molecules as red spheres, Measurements in orange]] |
| - | This evidence of convergent evolution between FAAH and other amidase signature enzymes supports the [http://euch6f.chem.emory.edu/burgidunitz.html Bürgi-Dunitz theory]. The Bürgi-Dunitz theory proposes that nucleophiles tend to follow a specific trajectory when attacking a carbonyl, resulting in many enzyme mechanisms having the same angle between an incoming nucleophile and the carbonyl it attacks. Water molecules in the active sites of enzymes are specifically positioned to force the nucleophile to approach at the exact [http://3.bp.blogspot.com/-NvsQyVPnLIw/UO91-BQTVgI/AAAAAAAAExw/-seGZcjU3DE/s400/burgi-duntz+trajectory.png "Bürgi-Dunitz angle"] of 107°. The determination that FAAH also has water molecules in its active site, helping the nucleophile to attack the amide carbonyl at a specific angle, adds additional support to the Bürgi-Dunitz theory <ref name="3LJ6"/>. | + | This evidence of convergent evolution between FAAH and other amidase signature enzymes supports the [http://euch6f.chem.emory.edu/burgidunitz.html Bürgi-Dunitz theory]. The Bürgi-Dunitz theory proposes that nucleophiles tend to follow a specific trajectory when attacking a carbonyl, resulting in many enzyme mechanisms having the same angle between an incoming nucleophile and the carbonyl it attacks [http://www.nature.com/nrd/journal/v11/n4/images/nrd3673-f4.jpg (FAAH bound to inhibitor)]. Water molecules in the active sites of enzymes are specifically positioned to force the nucleophile to approach at the exact [http://3.bp.blogspot.com/-NvsQyVPnLIw/UO91-BQTVgI/AAAAAAAAExw/-seGZcjU3DE/s400/burgi-duntz+trajectory.png "Bürgi-Dunitz angle"] of 107°. The determination that FAAH also has water molecules in its active site, helping the nucleophile to attack the amide carbonyl at a specific angle, adds additional support to the Bürgi-Dunitz theory <ref name="3LJ6"/>. |
</StructureSection> | </StructureSection> | ||
==Applications== | ==Applications== | ||
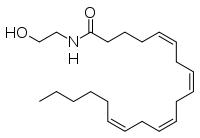
| - | The human nervous system has several types of chemical messengers, including amino acids, lipids, peptide hormones, and [http://en.wikipedia.org/wiki/Monoamine_neurotransmitter monoamines] <ref name="1MT5"/>. FAAH primarily degrades [http://www.chm.bris.ac.uk/motm/anandamide/ananh.htm anandamide] (AEA), a naturally-occurring signaling lipid that functions in the brain. AEA brings pain relief to the body. Inhibiting FAAH would likely sustain AEA signaling, leading to prolonged pain relief and decreased inflammation <ref name="2WAP"/>. | + | The human nervous system has several types of chemical messengers, including amino acids, lipids, peptide hormones, and [http://en.wikipedia.org/wiki/Monoamine_neurotransmitter monoamines] <ref name="1MT5"/>. FAAH primarily [http://www.scripps.edu/cravatt/images/faah_cartoon.gif degrades] [http://www.chm.bris.ac.uk/motm/anandamide/ananh.htm anandamide] (AEA), a naturally-occurring signaling lipid that functions in the brain. AEA brings [http://www.painresearchforum.org/news/11548-flat-tening-pain pain relief] to the body. Inhibiting FAAH would likely sustain AEA signaling, leading to prolonged pain relief and decreased inflammation <ref name="2WAP"/>. |
[[Image:AEA image.png|200 px|left|thumb|[http://en.wikipedia.org/wiki/File:Anandamide_skeletal.svg Figure 4: Anandamide]]] | [[Image:AEA image.png|200 px|left|thumb|[http://en.wikipedia.org/wiki/File:Anandamide_skeletal.svg Figure 4: Anandamide]]] | ||
| - | FAAH plays a role in endocannabinoid signaling that has intriguing potential as a drug target. This signaling system consists of endocannabinoid ligands (such as AEA), two G protein-coupled receptors (CB1 and CB2), and the enzymes that synthesize and degrade (such as FAAH) the signaling lipids. Previous research has explored the potential of regulating endocannabinoid signaling through the CB1 and CB2 receptors. However, molecules found to activate these receptors (such as [http://www.ch.ic.ac.uk/vchemlib/mim/bristol/thc/thc_text.htm tetrahydrocannabinol] (THC), the main psychoactive ingredient of [http://en.wikipedia.org/wiki/Cannabis_(drug) marijuana]), while providing the intended pain relief, also produce many undesirable side effects, such as decreased cognition and motor control. On the other hand, research involving FAAH inhibitors has shown that blocking this part of the pathway reduces pain without the unwanted side effects seen through CB1/CB2 activation. Thus, exploring the possibility of using FAAH inhibition to decrease pain relief with minimal side effects could lead to new pain treatment solutions <ref name="2WAP"/>. | + | FAAH plays a role in endocannabinoid signaling that has intriguing potential as a drug target. This signaling system consists of endocannabinoid ligands (such as AEA), two G protein-coupled receptors (CB1 and CB2), and the enzymes that synthesize and degrade (such as FAAH) the signaling lipids. Previous research has explored the potential of regulating endocannabinoid signaling through the CB1 and CB2 receptors. However, molecules found to activate these receptors (such as [http://www.ch.ic.ac.uk/vchemlib/mim/bristol/thc/thc_text.htm tetrahydrocannabinol] (THC), the main psychoactive ingredient of [http://en.wikipedia.org/wiki/Cannabis_(drug) marijuana]), while providing the intended pain relief, also produce many undesirable side effects, such as decreased cognition and motor control. On the other hand, research involving FAAH inhibitors has shown that blocking this part of the pathway reduces pain without the unwanted side effects seen through CB1/CB2 activation. Thus, exploring the possibility of using FAAH inhibition to decrease pain relief with minimal side effects could lead to new pain treatment solutions. For example, the [http://drugdiscoveryopinion.com/2009/04/pf-3845-%E2%80%93-a-potent-and-selective-faah-inhibitor/ PF-3845 inhibitor]of FAAH is extremely selective for the enzyme and raises AEA levels for a prolonged duration of time. Therefore, this inhibitor could be explored as a possible pharmaceutical product to target FAAH when extended pain relief is desired <ref name="2WAP"/>. |
| - | + | ||
Current revision
| This Sandbox is Reserved from Jan 06, 2014, through Aug 22, 2014 for use by the Biochemistry II class at the Butler University at Indianapolis, IN USA taught by R. Jeremy Johnson. This reservation includes Sandbox Reserved 911 through Sandbox Reserved 922. |
To get started:
More help: Help:Editing |
| |||||||||||
Applications
The human nervous system has several types of chemical messengers, including amino acids, lipids, peptide hormones, and monoamines [1]. FAAH primarily degrades anandamide (AEA), a naturally-occurring signaling lipid that functions in the brain. AEA brings pain relief to the body. Inhibiting FAAH would likely sustain AEA signaling, leading to prolonged pain relief and decreased inflammation [2].
FAAH plays a role in endocannabinoid signaling that has intriguing potential as a drug target. This signaling system consists of endocannabinoid ligands (such as AEA), two G protein-coupled receptors (CB1 and CB2), and the enzymes that synthesize and degrade (such as FAAH) the signaling lipids. Previous research has explored the potential of regulating endocannabinoid signaling through the CB1 and CB2 receptors. However, molecules found to activate these receptors (such as tetrahydrocannabinol (THC), the main psychoactive ingredient of marijuana), while providing the intended pain relief, also produce many undesirable side effects, such as decreased cognition and motor control. On the other hand, research involving FAAH inhibitors has shown that blocking this part of the pathway reduces pain without the unwanted side effects seen through CB1/CB2 activation. Thus, exploring the possibility of using FAAH inhibition to decrease pain relief with minimal side effects could lead to new pain treatment solutions. For example, the PF-3845 inhibitorof FAAH is extremely selective for the enzyme and raises AEA levels for a prolonged duration of time. Therefore, this inhibitor could be explored as a possible pharmaceutical product to target FAAH when extended pain relief is desired [2].
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Bracey MH, Hanson MA, Masuda KR, Stevens RC, Cravatt BF. Structural adaptations in a membrane enzyme that terminates endocannabinoid signaling. Science. 2002 Nov 29;298(5599):1793-6. PMID:12459591 doi:10.1126/science.1076535
- ↑ 2.0 2.1 2.2 2.3 Ahn K, Johnson DS, Mileni M, Beidler D, Long JZ, McKinney MK, Weerapana E, Sadagopan N, Liimatta M, Smith SE, Lazerwith S, Stiff C, Kamtekar S, Bhattacharya K, Zhang Y, Swaney S, Van Becelaere K, Stevens RC, Cravatt BF. Discovery and characterization of a highly selective FAAH inhibitor that reduces inflammatory pain. Chem Biol. 2009 Apr 24;16(4):411-20. PMID:19389627 doi:10.1016/j.chembiol.2009.02.013
- ↑ 3.0 3.1 3.2 3.3 Mileni M, Johnson DS, Wang Z, Everdeen DS, Liimatta M, Pabst B, Bhattacharya K, Nugent RA, Kamtekar S, Cravatt BF, Ahn K, Stevens RC. Structure-guided inhibitor design for human FAAH by interspecies active site conversion. Proc Natl Acad Sci U S A. 2008 Sep 2;105(35):12820-4. Epub 2008 Aug 27. PMID:18753625
- ↑ Mileni M, Garfunkle J, DeMartino JK, Cravatt BF, Boger DL, Stevens RC. Binding and inactivation mechanism of a humanized fatty acid amide hydrolase by alpha-ketoheterocycle inhibitors revealed from cocrystal structures. J Am Chem Soc. 2009 Aug 5;131(30):10497-506. PMID:19722626 doi:10.1021/ja902694n
- ↑ 5.0 5.1 5.2 Mileni M, Kamtekar S, Wood DC, Benson TE, Cravatt BF, Stevens RC. Crystal structure of fatty acid amide hydrolase bound to the carbamate inhibitor URB597: discovery of a deacylating water molecule and insight into enzyme inactivation. J Mol Biol. 2010 Jul 23;400(4):743-54. Epub 2010 May 21. PMID:20493882 doi:10.1016/j.jmb.2010.05.034