Introduction
Peptidoglycan transpeptidase (TP) also known as penicillin-binding proteins (PBP), are essential for bacterial cell wall synthesis and catalyze the cross-linking of peptidoglycan polymers during bacterial wall synthesis.Beta-lactam antibiotic, which includes the penicillins,cephalosporins,carbapenems, and the monobactam aztreonam (figure 1); bind and irreversibly inhibit the active site of TP. The overuse and misuse of b-lactam antibiotics has led to strains of Staphylococcus aureus (S.aureus) that are resistant to all currently available b-lactams and are often susceptible to so-called "last resort antibiotics", such as vancomycin.
Bacterial Cell Wall Structure
The bacterial cell wall is crucial for maintaining the structural integrity of bacteria and protects bacteria from osmotic stress and toxic compound. The cell wall is composed of peptidoglycan (Figure 2), and in Gram positive bacterial species (e.g. S. aureus) is many layers thick, while in Gram negative bacterial species (e.g. Escherichia coli) is only a few layers thick. The difference in the number of peptidoglycan layers accounts for the differential staining of these two groups of organisms. Peptidoglycan consists of a carbohydrate portion: alternating residues of N-acetylmuramic Acid (NAM) and N-acetylglucosamine (NAG) that polymerize to form long chains, and a protein portion: a pentapeptide chain that terminates with to D-alanines (D-Ala) residues. The pentapeptide chains are covalently bound to each NAM residue. Rows of peptidoglycan are cross-linked together with pentaglycine chains to form a "mesh-like" structure. This cross-linking reaction is catalyzed by TPs.
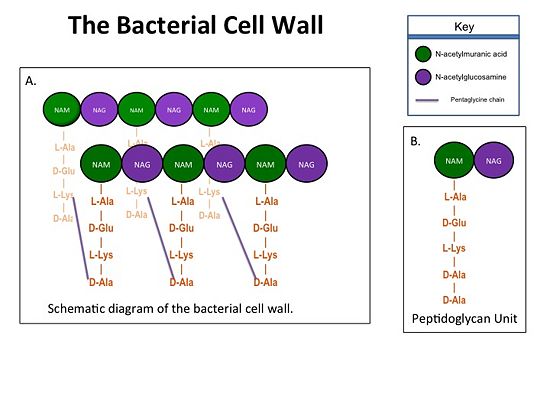
Figure 1.(A)Rows of Peptidoglycans forming a Bacterial Cell Wall (B)Peptidoglycan with D-Ala-D-Ala substrate
Catalytic Mechanism of Action of Transpeptidases
(a)The D-Ala side-chain substrate accesses the TP active site.
(b)The active site serine residue nucleophilically attacks the peptide bond between the terminal D-Ala residues. Terminal D-Ala residue exits the active site, and the remaining D-Ala residue forms a covalent bond with the active site serine residue to form an acyl-TP complex.
(c)Subsequently, a pentaglycine chain enters the TP active site through nucleophillic attack forms a covalent bond with the D-Ala formerly bound to the active site serine residue. As a result, the TP site serine residue is regenerated. The entire process takes approximately 4 seconds.
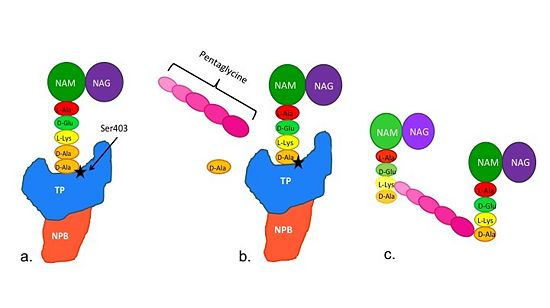
Figure 2. Schematic showing Catalytic Mechanism of PBP2a
Mechanism of Action of B-Lactam Antibiotics
The B-Lactam antibiotics inhibit bacterial cell growth by irreversibly inhibiting TP's and, therefore, bacterial cell wall sythesis. Specifically, B-Lactams are molecular mimics of a portion of the peptidoglycan polymer, namely the D-Ala-D-Ala moiety, which is the normal TP enzymatic substrate(Figure 4). As a result, bacterial TP enzymes are "tricked" into reacting with B-Lactams. Additionally, the B-Lactams are very reactive molecules due to their B-lactam ring, and readily react with the TP active site serine residue and sterically block the active site preventing the entry of nucleophiles that regenerate the active site serine residue such as the pentaglycine chain or water.
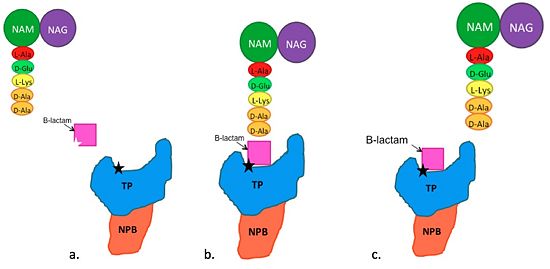
Figure 4. Schematic showing Mechanism of Action of B-Lactam Antibiotics. (a) The B-Lactam targets the active site, which normally is targeted by the pentaglycine. (b) The B-Lactam enters the active site and binds to the Ser403 thereby inhibiting the interaction between Ser403 and the pentaglycine. (c) The pentaglycine leaves the active site.
Structure of PBP2a, a B-Lactam Resistant Transpeptidase
Isolates of methicillin-resistant S. aureus (MRSA) are resistant to almost all currently available B-lactams because they have acquired an alternative PBP, PBP2A (encoded by the mecA gene) that is neither bound nor inhibited by B-lactams. PBP2a is composed of two domains: a non-penicillin binding domain and a transpeptidase-binding domain. The NBP domain of PBP2a is anchored in the cell membrane, while the TP domain residues in the periplasm with its active site facing the inner surface of the cell wall. The active site contains , which catalyzes the cross-linking of the peptidoglycan rows with pentaglycine cross-links.
B-Lactams that Inhibit PBP2a
MRSA becomes resistant to almost all B-Lactams by acquiring an alternative TP, PBP2a, that is neither bound nor inhibited by B-Lactams. Recently, two cephlosporins- and ceftaroline- that have anti-MRSA activity have been developed. Ceftobiprole is able to inhibit PBP2a because additional chemical groups at the position of ceftobiprole are able to interact with additional amino acid residues in PBP2a; specifically . As such, ceftobiprole is (shown as colors of the atom types CPK) is able to more efficiently react with Ser403 and therefore inhibit the activity of PBP2a.
PBP2a and Ceftaroline
(PDB:3ZFZ)
In addition to TP domain of PBP2a, there is an allosteric domain,highlighted orange, in which the distance between is 60Å.
Allosteric site serves as a binding site for the substrate . When the substrate binds to the (Tyr105, Asn146, Asp295, Tyr297), a conformational change occurs at the active site, opening it and allowing catalytic action to occur.
The medicine, , mimics the substrate at the opening the active site, allowing to .



