We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 934
From Proteopedia
(Difference between revisions)
| (30 intermediate revisions not shown.) | |||
| Line 13: | Line 13: | ||
oncostatin M, cardiotropin 1 (CT-1) and cardiotrophin-like cytokine (CLC) <ref name="Wen2012">PMID: 22182585</ref>. | oncostatin M, cardiotropin 1 (CT-1) and cardiotrophin-like cytokine (CLC) <ref name="Wen2012">PMID: 22182585</ref>. | ||
| - | <StructureSection load='1CNT_trunc.pdb' size=' | + | <StructureSection load='1CNT_trunc.pdb' size='500' frame='true' side='right' caption='Dimeric structure of CNTF in 2.4 Å resolution' scene='57/579704/Dimer_basic_view/3' > |
==Receptor for CNTF and the biological role of CNTF== | ==Receptor for CNTF and the biological role of CNTF== | ||
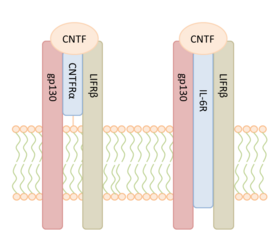
| + | [[Image:CNTF_receptorbinding.png|280px|right|thumb| Binding of CNTF to the high affinity receptor complexes CNTFRα/gp130/LIFRβ and to the low affinity receptor complex IL-6R/gp130/LIFRβ (modified from <ref name="Wen2012" /> <ref name="Li2011"> doi:10.1371/journal.pone.0023148 </ref>)]] | ||
CNTF exerts its biological function by binding into a tripartite | CNTF exerts its biological function by binding into a tripartite | ||
receptor complex consisting of a specific CNTF receptor subunit α (CNTFRα) linked to | receptor complex consisting of a specific CNTF receptor subunit α (CNTFRα) linked to | ||
| Line 43: | Line 44: | ||
neuron deficits <ref name="Sleeman2000" />, suggesting that CNTFRα might have a second ligand. | neuron deficits <ref name="Sleeman2000" />, suggesting that CNTFRα might have a second ligand. | ||
| - | < | + | ==Disease and Clinical applications== |
| + | A frameshift mutation in exon 2 of the CNTF gene affecting some 2-3 % of the population <ref name="Takahashi1994">PMID: 8075647 </ref> causes earlier onset of the disease and quicker declination of motor neuron function in MS patients <ref name="Giess2002">PMID: 11890844 </ref>. | ||
| + | MS patients with functional CNTF show 1,7-fold increase of CNTF mRNA expression in the cortex, suggesting CNTF secretion as an innate response to progressing neuronal damage <ref name="Dutta2007">PMID: 17898009 </ref>. | ||
| + | |||
| + | While not directly linked to disease, CNTF has been shown to inhibit the secretion of vascular endothelial growth factor (VEGF). VEGF, in turn, is upregulated in many diseases of the retina, causing increased angiogenesis. Thus CNTF-induced downregulation alleviates the symptoms of many ocular diseases <ref name="Li2011" />. It has been proposed that treatment with CNTF could counteract vision loss caused by age-related macular degeneration, retinitis pigmentosa and retinitis pigmentosa linked to Usher syndrome. In these cases the alleviating effect is produced by CNTF inducing regeneration of outer segments of cone cells in the retina <ref name=Li2010> PMID: 20209167 </ref>. Using encapsulated cells transfected with the human CNTF gene has been studied as a delivery method in clinical studies<ref name="Sieving"> doi: 10.1073/pnas.0600236103 </ref>, <ref name=Talcott2011> PMID: 21087953 </ref>. | ||
| + | As CNTF has also been shown to affect energy balance, it also has potential clinical applications in the prevention and treatment of obesity and type II diabetes <ref name="Sleeman2000" /> <ref name="Ettinger2003"> PMID: 12684362 </ref>. | ||
==Structure== | ==Structure== | ||
| - | Overview | ||
| - | Like with many other cytokines, the tertiary structure of CNTF consists of four anti-parallel α-helices | + | Like with many other cytokines, the tertiary structure of CNTF consists of four anti-parallel α-helices <scene name='57/579704/A-helix/4'>A</scene> (Arg<sup>13</sup>–His<sup>41</sup>), <scene name='57/579704/B-helix/2'>B</scene> (Glu<sup>69</sup>–Val<sup>96</sup>), <scene name='57/579704/C-helix/2'>C</scene> (Phe<sup>105</sup>–Leu<sup>129</sup>) and <scene name='57/579704/D-helix/2'>D</scene> (Phe<sup>152</sup>–Ser<sup>180</sup>), where helices A-B and C-D are connected |
by two cross-over loops and helices B-C by one short loop. A partial crystal structure of a truncated form of hCNTF (2-187) is displayed here <ref name="McDonald1995" />. | by two cross-over loops and helices B-C by one short loop. A partial crystal structure of a truncated form of hCNTF (2-187) is displayed here <ref name="McDonald1995" />. | ||
| - | The CNTFRα-binding surface epitope of CNTF was identified to consist of residues Arg<sup>25</sup>, Arg<sup>28</sup>, Gln<sup>63</sup>, Trp<sup>64</sup>, Gln<sup>74</sup>, Asp<sup>175</sup> and Arg<sup>177</sup>. These residues are located in helix A, the loop between helices A-B, helix B and helix D, and are <scene name='57/579704/Cntfr_binding_epitope_spacefil/1'>spatially clustered and surface accessible</scene> <ref name=”Panayotatos1995”> PMID: 7539796 </ref>. | + | The CNTFRα-binding surface epitope of CNTF was studied with random mutagenesis and identified to consist of residues Arg<sup>25</sup>, Arg<sup>28</sup>, Gln<sup>63</sup>, Trp<sup>64</sup>, Gln<sup>74</sup>, Asp<sup>175</sup> and Arg<sup>177</sup>. These residues are located in helix A, the loop between helices A-B, helix B and helix D, and are <scene name='57/579704/Cntfr_binding_epitope_spacefil/1'>spatially clustered and surface accessible</scene> <ref name=”Panayotatos1995”> PMID: 7539796 </ref>. |
In addition, the LIFR-binding epitope of CNTF was identified to consist of <scene name='57/579704/Lifr_binding_epitope/1'>amino acid chains</scene> Glu<sup>36</sup>-Met<sup>56</sup>, Leu<sup>91</sup>-Ile<sup>109</sup> and Gly<sup>147</sup>-Leu<sup>162</sup> <ref name=”Kallen1999”> PMID: 10207005</ref>. The ability of hCNTF to bind both to CNTFRα and IL-6R has been mapped to | In addition, the LIFR-binding epitope of CNTF was identified to consist of <scene name='57/579704/Lifr_binding_epitope/1'>amino acid chains</scene> Glu<sup>36</sup>-Met<sup>56</sup>, Leu<sup>91</sup>-Ile<sup>109</sup> and Gly<sup>147</sup>-Leu<sup>162</sup> <ref name=”Kallen1999”> PMID: 10207005</ref>. The ability of hCNTF to bind both to CNTFRα and IL-6R has been mapped to | ||
residue Gln<sup>63</sup> <ref name="Schuster2003" />. | residue Gln<sup>63</sup> <ref name="Schuster2003" />. | ||
| - | + | The dimeric structure of CNTF is attributed to the properties of hydrophobic (Leu<sup>91</sup>, Leu<sup>113</sup>, Leu<sup>114</sup>, Ala<sup>117</sup>, Tyr<sup>121</sup>, Ile<sup>128</sup>), polar (Gln<sup>95</sup>) and charged residues (Arg<sup>81</sup>, His<sup>84</sup>, Glu<sup>92</sup>, His<sup>106</sup>, His<sup>110</sup>, Glu<sup>124</sup>, Glu<sup>125</sup>) in the B and C helices of monomeric CNTF. These interface sites are highly conserved among species, except for Gln<sup>95</sup>. In the dimeric structure, <scene name='57/579704/Cntf_hbonds/3'>two water molecules are buried in the interface of the two monomers</scene>, forming hydrogen bonds to His<sup>84</sup> and Tyr<sup>121</sup> of each monomer<ref name="McDonald1995">PMID: 7796798</ref>. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | the | + | |
| - | + | ||
| - | (<ref name="McDonald1995">PMID: 7796798</ref>) Two water molecules are buried at the interface which form hydrogen bonds to the side chain of His84 and Tyrl21 (Figure IC). | ||
| - | Nearly all interface side chains are conserved amongst CNTF sequences from | ||
| - | different species (except Gln95).<scene name='57/579704/Cntf_dimer_buriedwater/2'>Hydrogen bond-forming buried water molecules</scene> | ||
| - | |||
| - | ==Disease and Clinical applications== | ||
| - | A frameshift mutation in exon 2 of the CNTF gene affecting some 2-3 % of the population <ref name="Takahashi1994">PMID: 8075647 </ref> causes earlier onset of the disease and quicker declination of motor neuron function in MS patients <ref name="Giess2002">PMID: 11890844 </ref>. | ||
| - | MS patients with functional CNTF show 1,7-fold increase of CNTF mRNA expression in the cortex, suggesting CNTF secretion as an innate response to progressing neuronal damage <ref name="Dutta2007">PMID: 17898009 </ref>. | ||
| - | |||
| - | While not directly linked to disease, CNTF has been shown to inhibit the secretion of VEGF in the human retina, alleviating the symptoms of some ocular diseases <ref name ''Li2011''> doi:10.1371/journal.pone.0023148 </ref>. It has been proposed that treatment with CNTF could counteract vision loss caused by age-related macular degeneration, retinitis pigmentosa and retinitis pigmentosa linked to Usher syndrome. In these cases the alleviating effect is produced by CNTF inducing regeneration of outer segments of cone cells in the retina <ref name=Li2010> PMID: 20209167 </ref>. Using encapsulated cells transfected with the human CNTF gene has been studied as a delivery method in clinical studies<ref name="Sieving"> doi: 10.1073/pnas.0600236103 </ref>, <ref name=Talcott2011> PMID: 21087953 </ref>. | ||
| - | As CNTF has also been shown to affect energy balance, it also has potential clinical applications in the prevention and treatment of obesity and type II diabetes <ref name="Sleeman2000" /> <ref name="Ettinger2003"> PMID: 12684362 </ref>. | ||
</StructureSection> | </StructureSection> | ||
| Line 83: | Line 70: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| - | |||
| - | Richardson PM: Ciliary neurotrophic factor: A review. Pharmac Ther 63: 187-198 (1994). | ||
| - | |||
| - | |||
| - | Wen R, Song Y, Kjellstrom S, Tanikawa A, Liu Y, Li Y, Zhao L, Bush RA, Laties AM and Sieving PA: Regulation of rod phototransduction machinery by ciliary neurotrophic factor. J Neurosci 26: 13523-13530 (2006). | ||
Current revision
| This Sandbox is Reserved from 01/04/2014, through 30/06/2014 for use in the course "510042. Protein structure, function and folding" taught by Prof Adrian Goldman, Tommi Kajander, Taru Meri, Konstantin Kogan and Juho Kellosalo at the University of Helsinki. This reservation includes Sandbox Reserved 923 through Sandbox Reserved 947. |
To get started:
More help: Help:Editing |
Introduction
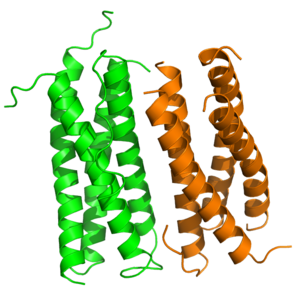
Ciliary neurotrophic factor, 1cnt
Human Ciliary Neurotrophic Factor (CNTF) is a roughly 23 kDa protein consisting of a single polypeptide chain of 200 amino acid residues. It is a nerve growth factor belonging to the Interleukin-6 (IL-6) family of neuropoietic cytokines. Other members of this family include leukemia inhibitory factor (LIF), IL-6, IL-11, oncostatin M, cardiotropin 1 (CT-1) and cardiotrophin-like cytokine (CLC) [1].
| |||||||||||
Additional Resources
References
- ↑ 1.0 1.1 1.2 Wen R, Tao W, Li Y, Sieving PA. CNTF and retina. Prog Retin Eye Res. 2012 Mar;31(2):136-51. doi: 10.1016/j.preteyeres.2011.11.005., Epub 2011 Dec 10. PMID:22182585 doi:http://dx.doi.org/10.1016/j.preteyeres.2011.11.005
- ↑ 2.0 2.1 Li R, Wen R, Banzon T, Maminishkis A, Miller SS. CNTF mediates neurotrophic factor secretion and fluid absorption in human retinal pigment epithelium. PLoS One. 2011;6(9):e23148. doi: 10.1371/journal.pone.0023148. Epub 2011 Sep 2. PMID:21912637 doi:http://dx.doi.org/10.1371/journal.pone.0023148
- ↑ Panayotatos N, Everdeen D, Liten A, Somogyi R, Acheson A. Recombinant human CNTF receptor alpha: production, binding stoichiometry, and characterization of its activity as a diffusible factor. Biochemistry. 1994 May 17;33(19):5813-8. PMID:8180210
- ↑ Davis S, Aldrich TH, Ip NY, Stahl N, Scherer S, Farruggella T, DiStefano PS, Curtis R, Panayotatos N, Gascan H, et al.. Released form of CNTF receptor alpha component as a soluble mediator of CNTF responses. Science. 1993 Mar 19;259(5102):1736-9. PMID:7681218
- ↑ 5.0 5.1 Schuster B, Kovaleva M, Sun Y, Regenhard P, Matthews V, Grotzinger J, Rose-John S, Kallen KJ. Signaling of human ciliary neurotrophic factor (CNTF) revisited. The interleukin-6 receptor can serve as an alpha-receptor for CTNF. J Biol Chem. 2003 Mar 14;278(11):9528-35. PMID:12643274
- ↑ 6.0 6.1 6.2 6.3 Cognet I, Guilhot F, Chevalier S, Guay-Giroux A, Bert A, Elson GC, Gascan H, Gauchat JF. Expression of biologically active mouse ciliary neutrophic factor (CNTF) and soluble CNTFRalpha in Escherichia coli and characterization of their functional specificities. Eur Cytokine Netw. 2004 Jul-Sep;15(3):255-62. PMID:15542451
- ↑ 7.0 7.1 7.2 7.3 Sleeman MW, Anderson KD, Lambert PD, Yancopoulos GD and Wiegand SJ. "The ciliary neurotrophic factor and its receptor, CNTFRα". Pharm Acta Helv 74: 265-272 (2000). http://dx.doi.org/10.1016/S0165-7208(00)80028-8
- ↑ Takahashi R, Yokoji H, Misawa H, Hayashi M, Hu J, Deguchi T. A null mutation in the human CNTF gene is not causally related to neurological diseases. Nat Genet. 1994 May;7(1):79-84. PMID:8075647 doi:http://dx.doi.org/10.1038/ng0594-79
- ↑ Giess R, Maurer M, Linker R, Gold R, Warmuth-Metz M, Toyka KV, Sendtner M, Rieckmann P. Association of a null mutation in the CNTF gene with early onset of multiple sclerosis. Arch Neurol. 2002 Mar;59(3):407-9. PMID:11890844
- ↑ Dutta R, McDonough J, Chang A, Swamy L, Siu A, Kidd GJ, Rudick R, Mirnics K, Trapp BD. Activation of the ciliary neurotrophic factor (CNTF) signalling pathway in cortical neurons of multiple sclerosis patients. Brain. 2007 Oct;130(Pt 10):2566-76. PMID:17898009 doi:http://dx.doi.org/10.1093/brain/awm206
- ↑ Li Y, Tao W, Luo L, Huang D, Kauper K, Stabila P, Lavail MM, Laties AM, Wen R. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PLoS One. 2010 Mar 2;5(3):e9495. doi: 10.1371/journal.pone.0009495. PMID:20209167 doi:http://dx.doi.org/10.1371/journal.pone.0009495
- ↑ Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006 Mar 7;103(10):3896-901. Epub 2006 Feb 27. PMID:16505355 doi:http://dx.doi.org/10.1073/pnas.0600236103
- ↑ Talcott KE, Ratnam K, Sundquist SM, Lucero AS, Lujan BJ, Tao W, Porco TC, Roorda A, Duncan JL. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Invest Ophthalmol Vis Sci. 2011 Apr 6;52(5):2219-26. doi: 10.1167/iovs.10-6479. PMID:21087953 doi:http://dx.doi.org/10.1167/iovs.10-6479
- ↑ Ettinger MP, Littlejohn TW, Schwartz SL, Weiss SR, McIlwain HH, Heymsfield SB, Bray GA, Roberts WG, Heyman ER, Stambler N, Heshka S, Vicary C, Guler HP. Recombinant variant of ciliary neurotrophic factor for weight loss in obese adults: a randomized, dose-ranging study. JAMA. 2003 Apr 9;289(14):1826-32. PMID:12684362 doi:http://dx.doi.org/10.1001/jama.289.14.1826
- ↑ 15.0 15.1 McDonald NQ, Panayotatos N, Hendrickson WA. Crystal structure of dimeric human ciliary neurotrophic factor determined by MAD phasing. EMBO J. 1995 Jun 15;14(12):2689-99. PMID:7796798
- ↑ Panayotatos N, Radziejewska E, Acheson A, Somogyi R, Thadani A, Hendrickson WA, McDonald NQ. Localization of functional receptor epitopes on the structure of ciliary neurotrophic factor indicates a conserved, function-related epitope topography among helical cytokines. J Biol Chem. 1995 Jun 9;270(23):14007-14. PMID:7539796
- ↑ Kallen KJ, Grotzinger J, Lelievre E, Vollmer P, Aasland D, Renne C, Mullberg J, Myer zum Buschenfelde KH, Gascan H, Rose-John S. Receptor recognition sites of cytokines are organized as exchangeable modules. Transfer of the leukemia inhibitory factor receptor-binding site from ciliary neurotrophic factor to interleukin-6. J Biol Chem. 1999 Apr 23;274(17):11859-67. PMID:10207005