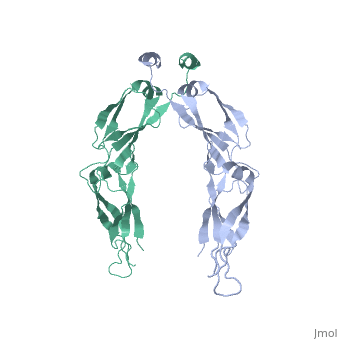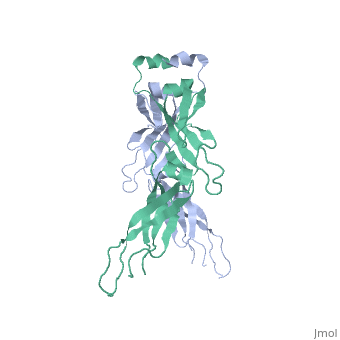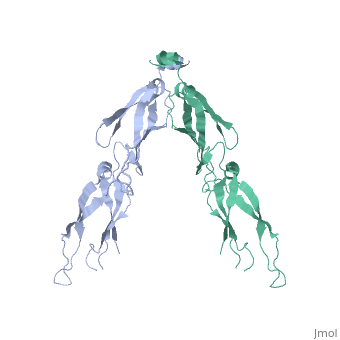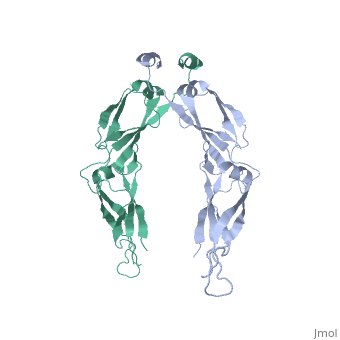We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Hsp40
From Proteopedia
(Difference between revisions)
(New page: =='Hsp40'== <StructureSection load='2QLD' size='340' side='right' caption='Human Hsp40 Hdj1' scene=''> Hsp40 ( J protein) and NEF cochaperones regulate the Hsp70 reaction cycle. The Hsp40...) |
|||
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | ==' | + | =='Hsp40s'== |
| - | <StructureSection load='2QLD' size='340' side='right' caption='Human Hsp40 Hdj1' scene=''> | + | <StructureSection load='2QLD' size='340' side='right' caption='Human Hsp40 Hdj1 (PDB code [[2qld]]).' scene=''> |
| - | + | These proteins ( J protein) and NEF cochaperones regulate the Hsp70 reaction cycle. The Hsp40 proteins constitute a large family with more than 40 members in | |
humans. All of them contain a J domain, which binds to the N-terminal ATPase domain of Hsp70 and the adjacent linker region. Canonical Hsp40s (members of classes I and II) function as chaperones independently and recruit Hsp70 to nonnative substrate proteins. Other Hsp40s (class III) are more diverse | humans. All of them contain a J domain, which binds to the N-terminal ATPase domain of Hsp70 and the adjacent linker region. Canonical Hsp40s (members of classes I and II) function as chaperones independently and recruit Hsp70 to nonnative substrate proteins. Other Hsp40s (class III) are more diverse | ||
and combine the J domain with a variety of functional modules. The interaction with Hsp70 strongly stimulates the hydrolysis of Hsp70-bound ATP to | and combine the J domain with a variety of functional modules. The interaction with Hsp70 strongly stimulates the hydrolysis of Hsp70-bound ATP to | ||
Current revision
'Hsp40s'
| |||||||||||