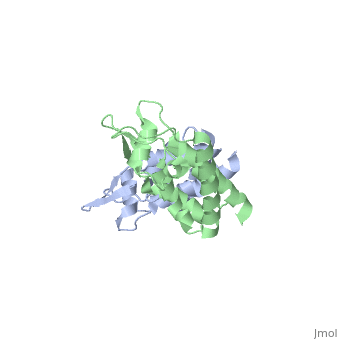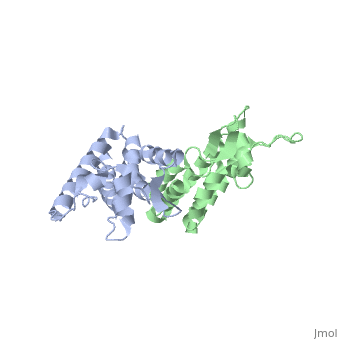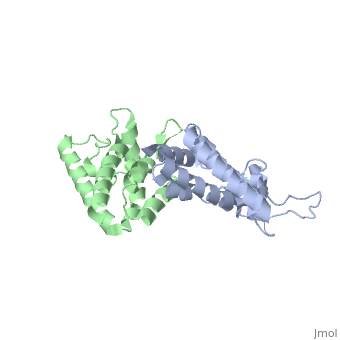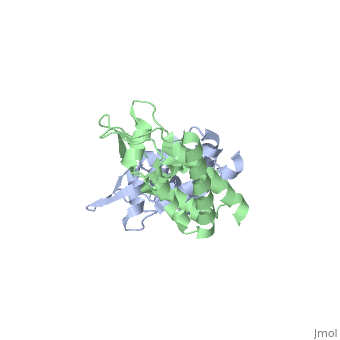
We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Gag polyprotein
From Proteopedia
(Difference between revisions)
| (8 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='2wlv' size='350' side='right' caption='Gag polyprotein N-terminal capsid domain of HIV-2 (PDB entry [[2wlv]])' scene=''> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| + | == Function == | ||
The '''Gag polyprotein''' (Gag) is part of the basic infrastructure of retroviruses. The Gag is processed during maturation to '''matrix protein (MA)''', '''capsid protein (CA)''', '''spacer peptides (SP1, SP2)''', '''nucleocapsid protein (NC)''' and '''p6'''. For detailed discussion of HIV-1 Gag polyprotein see [[Hiv-1 gag]]. | The '''Gag polyprotein''' (Gag) is part of the basic infrastructure of retroviruses. The Gag is processed during maturation to '''matrix protein (MA)''', '''capsid protein (CA)''', '''spacer peptides (SP1, SP2)''', '''nucleocapsid protein (NC)''' and '''p6'''. For detailed discussion of HIV-1 Gag polyprotein see [[Hiv-1 gag]]. | ||
| Line 36: | Line 8: | ||
==Capsid (CA) Domain== | ==Capsid (CA) Domain== | ||
| - | Overall, the mature CA<sup>N</sup> domain is very similar in structure to the corresponding domain of the immature Gag<sup>283</sup> polyprotein | + | Overall, the mature CA<sup>N</sup> domain is very similar in structure to the corresponding domain of the immature Gag<sup>283</sup> polyprotein. The CA<sup>N</sup> protein contains 7 α-helices (helix 1-helix 7) that pack together to form a triangular shape, which helps facilitate the final complex formation for the capsid core particles. There are two significant structural differences between the immature and mature versions of the CA<sup>N</sup> domain: an N-terminal β-hairpin and a 2-Angstrom displacement of helix 6. |
In the mature CA<sup>N</sup> protein, the N-terminal residues form an anti-parallel β-hairpin instead of the random coil that is observed when the same residues are compared in the immature Gag<sup>283</sup> polyprotein. The NH<sub>2</sub>+ group of the N-terminus proline establishes a salt bridge with a nearby aspartic acid, which stabilizes the β-hairpin. This N-terminal β-hairpin is required for the final formation of the viral capsid, and many studies have shown through conservation and mutagenesis that this β-hairpin is responsible for the stabilization of the protein complexes involved in the capsid formation <ref name="gitti">PMID:8662505</ref><ref name="von">PMID:9501077</ref>. | In the mature CA<sup>N</sup> protein, the N-terminal residues form an anti-parallel β-hairpin instead of the random coil that is observed when the same residues are compared in the immature Gag<sup>283</sup> polyprotein. The NH<sub>2</sub>+ group of the N-terminus proline establishes a salt bridge with a nearby aspartic acid, which stabilizes the β-hairpin. This N-terminal β-hairpin is required for the final formation of the viral capsid, and many studies have shown through conservation and mutagenesis that this β-hairpin is responsible for the stabilization of the protein complexes involved in the capsid formation <ref name="gitti">PMID:8662505</ref><ref name="von">PMID:9501077</ref>. | ||
| Line 46: | Line 18: | ||
==3D structures of Gag polyprotein== | ==3D structures of Gag polyprotein== | ||
| + | [[Gag polyprotein 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
==Additional Resources== | ==Additional Resources== | ||
Current revision
| |||||||||||
Contents |
Additional Resources
For additional information, see: Human Immunodeficiency Virus
Reference
- ↑ Coffin, J., S. Hughes, and H. Varmus, Retroviruses. 1997: Cold Spring Harbor Laboratory Press.
- ↑ 2.0 2.1 Gitti RK, Lee BM, Walker J, Summers MF, Yoo S, Sundquist WI. Structure of the amino-terminal core domain of the HIV-1 capsid protein. Science. 1996 Jul 12;273(5272):231-5. PMID:8662505
- ↑ 3.0 3.1 von Schwedler UK, Stemmler TL, Klishko VY, Li S, Albertine KH, Davis DR, Sundquist WI. Proteolytic refolding of the HIV-1 capsid protein amino-terminus facilitates viral core assembly. EMBO J. 1998 Mar 16;17(6):1555-68. PMID:9501077 doi:10.1093/emboj/17.6.1555
- ↑ Braaten D, Franke EK, Luban J. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J Virol. 1996 Jun;70(6):3551-60. PMID:8648689
- ↑ Thali M, Bukovsky A, Kondo E, Rosenwirth B, Walsh CT, Sodroski J, Gottlinger HG. Functional association of cyclophilin A with HIV-1 virions. Nature. 1994 Nov 24;372(6504):363-5. PMID:7969495 doi:http://dx.doi.org/10.1038/372363a0
- ↑ Ackerson B, Rey O, Canon J, Krogstad P. Cells with high cyclophilin A content support replication of human immunodeficiency virus type 1 Gag mutants with decreased ability to incorporate cyclophilin A. J Virol. 1998 Jan;72(1):303-8. PMID:9420228
Team from University of Missouri, Columbia, MO
- Students: Zheng Wang, Allison Tegge, Xin Deng
- Advisors: Jianlin Cheng, PhD, Department of Computer Science, Informatics Institute, the Life Science Center, Interdisciplinary Plant Group, University of Missouri, Columbia
- Mentor: Chun Tang, PhD, Department of Biochemistry, University of Missouri, Columbia
NMR Equipment and the Authors
Created by Allison Tegge and David Canner