We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox 126
From Proteopedia
(Difference between revisions)
| (8 intermediate revisions not shown.) | |||
| Line 5: | Line 5: | ||
==='''Background Information'''=== | ==='''Background Information'''=== | ||
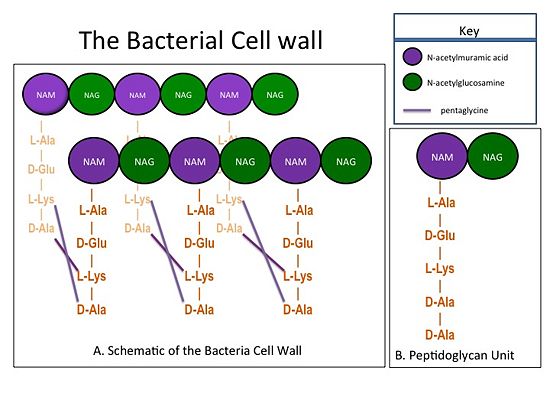
| - | The bacterial cell wall is composed of sheets of peptidoglycan cross-linked together to form a highly polymeric "mesh" that helps maintain the structural strength of the cell (Figure 1). A peptidoglycan sheet consists of alternating residues of <font color='purple'> '''N-acetylmuramic acid (NAM)''' </font> and <font color='green'> '''N-acetylglucosamine (NAG)''' </font> linked together by β-(1,4)- glycosidic bonds. In ''Staphylococcus aureus'' (S. aureus), the NAM residues are coupled to a (D-Ala) residues. The sheets of peptidoglycan are cross-linked together with pentaglycine chains. The cross-linking of adjacent peptidoglycan sheets is catalyzed by transpeptidases (TP). Beta-Lactam antibiotics, such as penicillin and the anti-MRSA cephlosporins, ceftobiprole and ceftaroline, stop the production of the cell wall, and so kill bacteria, by irreversibly inhibiting TPs. Therefore, TPs are also called penicillin-binding proteins.[[Image:CellWall.jpg|thumb|alt= Alt text| Figure 1.(A) This moiety is polymerized to form sheets of peptidoglycan. Adjacent sheets of peptidoglycan are cross-linked together by pentaglycine "bridges" to form a polymeric "mesh" that is essential for the structural integrity of the bacterial cell (B) The cell wall is composed of repeating units of a NAM/NAG disaccharide and peptide moiety; ''i.e.'', peptidoglycan |550px]] | + | The bacterial cell wall is composed of sheets of peptidoglycan cross-linked together to form a highly polymeric "mesh" that helps maintain the structural strength of the cell (Figure 1). A peptidoglycan sheet consists of alternating residues of <font color='purple'> '''N-acetylmuramic acid (NAM)''' </font> and <font color='green'> '''N-acetylglucosamine (NAG)''' </font> linked together by β-(1,4)- glycosidic bonds. In ''Staphylococcus aureus'' (S. aureus), the NAM residues are coupled to a (D-Ala) residues. The sheets of peptidoglycan are cross-linked together with pentaglycine chains. The cross-linking of adjacent peptidoglycan sheets is catalyzed by transpeptidases (TP). Beta-Lactam antibiotics, such as penicillin and the anti-MRSA cephlosporins, ceftobiprole and ceftaroline, stop the production of the cell wall, and so kill bacteria, by irreversibly inhibiting TPs. Therefore, TPs are also called penicillin-binding proteins.[[Image:CellWall.jpg|thumb|alt= Alt text| Figure 1.(A) This moiety is polymerized to form sheets of peptidoglycan. Adjacent sheets of peptidoglycan are cross-linked together by pentaglycine "bridges" to form a polymeric "mesh" that is essential for the structural integrity of the bacterial cell. (B) The cell wall is composed of repeating units of a NAM/NAG disaccharide and peptide moiety; ''i.e.'', peptidoglycan. |550px]] |
PBP2a is composed of two domains: <font color='orange'> '''a non-penicillin binding <scene name='37/372726/Npb/4'>(NPB)</scene> domain'''</font> (residues 27-326) and a <font color='dodgerblue'> '''transpeptidase''' </font> <scene name='37/372726/Tp/5'>(TP)</scene> domain (residues 327-668). The NBP domain of PBP2a is anchored in the cell membrane, while the TP domain "sits" in the periplasm with its active site facing the inner surface of the cell wall. The active site contains a serine residue position 403 <scene name='37/372726/Ser403/2'>(Ser403)</scene> which catalyzes the cross-linking of the peptidoglycan rows with pentaglycine cross-links. | PBP2a is composed of two domains: <font color='orange'> '''a non-penicillin binding <scene name='37/372726/Npb/4'>(NPB)</scene> domain'''</font> (residues 27-326) and a <font color='dodgerblue'> '''transpeptidase''' </font> <scene name='37/372726/Tp/5'>(TP)</scene> domain (residues 327-668). The NBP domain of PBP2a is anchored in the cell membrane, while the TP domain "sits" in the periplasm with its active site facing the inner surface of the cell wall. The active site contains a serine residue position 403 <scene name='37/372726/Ser403/2'>(Ser403)</scene> which catalyzes the cross-linking of the peptidoglycan rows with pentaglycine cross-links. | ||
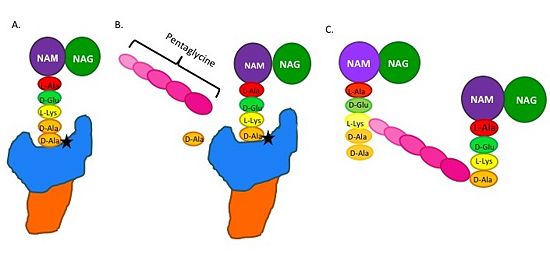
| - | ==='''Mechanism of | + | ==='''Catalytic Mechanism of Action of Transpeptidases'''=== |
| - | The beta-lactam antibiotics irreversibly bind to and inhibit TPs. This results in the disruption of peptidoglycan synthesis and ultimately cell growth. Specifically, beta-lactams, such as penicillin and the anti-MRSA | + | (A) The peptidoglycan D-Ala D-Ala moiety enters the TP active site, which is in the TP domain (blue). |
| + | |||
| + | (B) The active site serine residue (star) reacts with and breaks the peptide bond between the D-Ala residues. The terminal D-Ala residue exits the active site. The remaining D-Ala residue is covalently bound to the active site serine residue, and therefore, to TP. The incoming pentaglycine chain reacts with the bound D-Ala residue and is cross-linked to the D-Ala residue. | ||
| + | (C) This results in cross-linking between adjacent peptidoglycan "sheets" and regeneration of the active site serine residue so it can catalyze another cross-linking reaction.[[Image: MechanismofPBP.jpg|thumb|alt= Alt text|Figure 2.Schematic showing Catalytic Mechanism of PBP2a |550px]] | ||
| + | |||
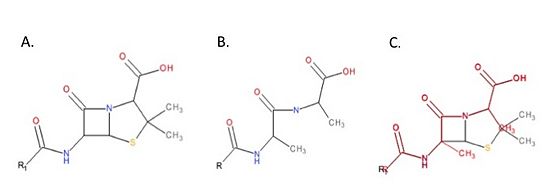
| + | ==='''Mechanism of Action of Beta-Lactam Antibiotics'''=== | ||
| + | The beta-lactam antibiotics irreversibly bind to and inhibit TPs. This results in the disruption of peptidoglycan synthesis and ultimately cell growth. Specifically, beta-lactams, such as penicillin and the anti-MRSA cephalosporins, ceftobiprole and ceftaroline, are molecular mimics of the peptidoglycan D-Ala-D-Ala moiety; the normal TP substrate (Figure 3; Tipper and Strominger, 1965). Therefore, they “trick” the TP active site serine residue to react with them, resulting in the irreversible inhibition of TP activity and of cell wall synthesis. | ||
| + | [[Image:DalMimic.jpg|thumb|alt= Alt text| Figure 3. Mechanism of action of beta-lactams. A. Chemical structure of penicillin, a beta-lactam antibiotic. B. Chemical structure of the peptidoglycan D-Ala-D-Ala moiety, the normal TP substrate. C. Superimposition of the D-Ala-D-Ala moiety (red) with penicillin (black). The beta-lactams mimic the structure of the D-Ala-D-Ala moiety of peptidoglycan. This ensures TPs react with beta-lactams.|550px]] | ||
| + | |||
==='''MRSA, PBP2a, and anti-MRSA Cephalosporins'''=== | ==='''MRSA, PBP2a, and anti-MRSA Cephalosporins'''=== | ||
MRSA becomes resistant to beta-lactams by acquiring an alternative TP, PBP2a, that is encoded by the ''mecA'' gene (Matsuhashi ''et al.'', 1986). PBP2a is compromised in its ability to react with beta-lactam; therefore, MRSA strains are resistant to beta-lactams and are able to make their cell wall in the presence of high concentrations of beta-lactams. | MRSA becomes resistant to beta-lactams by acquiring an alternative TP, PBP2a, that is encoded by the ''mecA'' gene (Matsuhashi ''et al.'', 1986). PBP2a is compromised in its ability to react with beta-lactam; therefore, MRSA strains are resistant to beta-lactams and are able to make their cell wall in the presence of high concentrations of beta-lactams. | ||
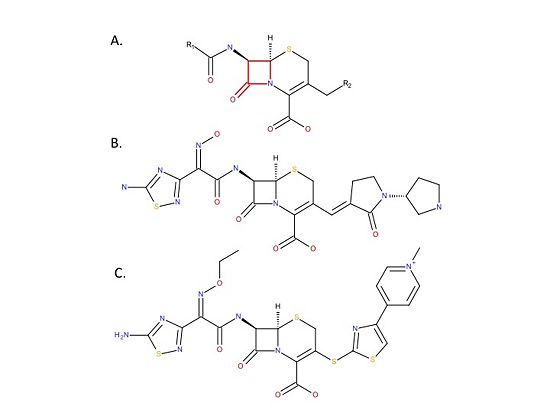
| - | Recently, two broad range cephalosporins: ceftaroline and ceftaroline (Figure), that have anti-MRSA activity because they bind and inhibit PBP2a have been developed. | + | Recently, two broad range cephalosporins: ceftaroline and ceftaroline (Figure 4), that have anti-MRSA activity because they bind and inhibit PBP2a have been developed.[[Image:MedicinesSchematic.jpg|thumb|alt= Alt text| Figure 4. Chemical structure of anti-MRSA cephalosporins. A. Chemical structure of the cephalosporin backbone. Cephalosporins are beta-lactam antibiotics that have a core backbone that includes a beta-lactam ring (red). There are many different cephalosporins, all of which have different antimicrobial activities and chemical properties. The differences between the cephalosporins is due to differences in the R1 and R2 groups. B. Chemical structure of ceftobiprole. C. Chemical structure of ceftaroline. |550px]] |
Current revision
| |||||||||||