Saposin
From Proteopedia
(Difference between revisions)
| (9 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
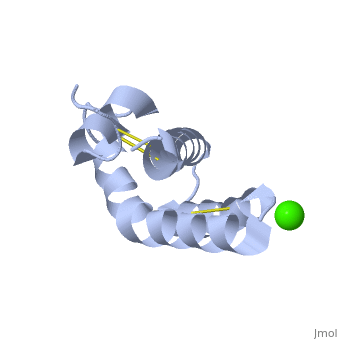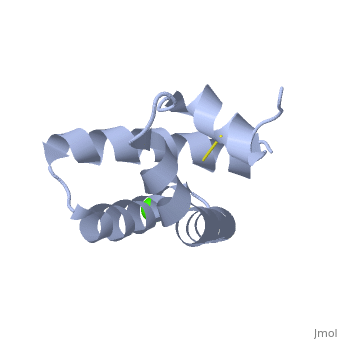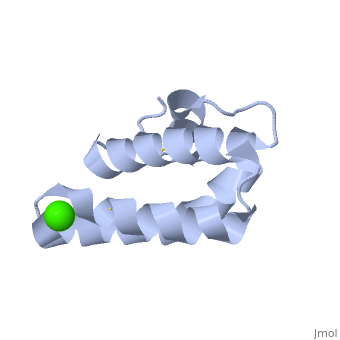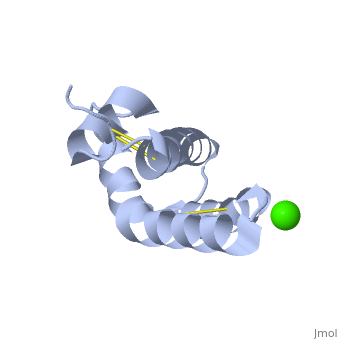
| - | + | <StructureSection load='2dob' size='350' side='right' scene='' caption='Human saposin A complex with Ca+2 [[2dob]]'> | |
| + | == Function == | ||
| + | '''Saposin''' (Sap) is a small protein which functions as activator of lipid-degrading enzymes. They act by isolating the lipid substrate from the membrane. Sap is synthesized as a precursor – prosaposin – which contain 4 SapB active domains (cleaved to saposin A,B,C and D) and 2 SapA domains which are cleaved off<ref>PMID:2001789</ref>. See also [[Lipid metabolism]]. | ||
| - | '''Saposin''' | + | *'''Saposin A and C''' stimulate hydrolysis of methylumbelliferyl β-galactoside by β-glucosylceramidase and of galactocerebrocide by β-galactosylceramidase<ref>PMID:2717620</ref>. For more details see [[Molecular Playground/Saposin C]].<br /> |
| + | *'''Saposin B''' facilitates lipid binding to CD1d<ref>PMID:17372201</ref>.<br /> | ||
| + | *'''Saposin D''' stimulates acid ceramidase activity<ref>PMID:8203897</ref>.<br /> | ||
| + | == Disease == | ||
| + | Mutations in saposin B are autosomal recessive trait resulting in clinical metachromatic leukodystrophy<ref>PMID:17616409</ref>. Mutations in saposin D cause urinary system defects<ref>PMID:15345707</ref>. | ||
| + | </StructureSection> | ||
== 3D Structures of Saposin == | == 3D Structures of Saposin == | ||
Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| - | + | *Saposin A residues 60-143 | |
| - | [[2dob]] – hSapA | + | **[[2dob]], [[4uex]] – hSapA – human<br /> |
| - | [[4ddj]] – hSapA + LDAO<br /> | + | **[[4ddj]] – hSapA + LDAO<br /> |
| + | **[[7p4d]] – mSapA – mouse<br /> | ||
| + | **[[5nxb]] – mSapA + galactocerebrosidase <br /> | ||
| - | + | *Saposin A residues 1-81 | |
| - | [[ | + | **[[6d80]] – hSapA + mitochondrial calcium uniporter<br /> |
| - | + | *Saposin B | |
| - | [[ | + | **[[1n69]] – hSapB + lipid<br /> |
| - | [[ | + | **[[4v2o]] – hSapB + chloroquine<br /> |
| - | + | *Saposin C | |
| - | [[ | + | **[[1m12]], [[1sn6]] – hSapC – NMR<br /> |
| - | [[ | + | **[[2gtg]], [[2qyp]], [[2z9a]] – hSapC residues 311-391 |
| + | *Saposin D | ||
| + | |||
| + | **[[3bqp]], [[3bqq]], [[2r1q]], [[2rb3]] - hSapD residues 405-484<br /> | ||
| + | **[[5u85]] - mSapD residues 438-519<br /> | ||
| + | }} | ||
| + | == References == | ||
| + | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
3D Structures of Saposin
Updated on 14-November-2023
References
- ↑ O'Brien JS, Kishimoto Y. Saposin proteins: structure, function, and role in human lysosomal storage disorders. FASEB J. 1991 Mar 1;5(3):301-8. PMID:2001789
- ↑ Morimoto S, Martin BM, Yamamoto Y, Kretz KA, O'Brien JS, Kishimoto Y. Saposin A: second cerebrosidase activator protein. Proc Natl Acad Sci U S A. 1989 May;86(9):3389-93. PMID:2717620
- ↑ Yuan W, Qi X, Tsang P, Kang SJ, Illarionov PA, Besra GS, Gumperz J, Cresswell P. Saposin B is the dominant saposin that facilitates lipid binding to human CD1d molecules. Proc Natl Acad Sci U S A. 2007 Mar 27;104(13):5551-6. Epub 2007 Mar 19. PMID:17372201 doi:http://dx.doi.org/10.1073/pnas.0700617104
- ↑ Azuma N, O'Brien JS, Moser HW, Kishimoto Y. Stimulation of acid ceramidase activity by saposin D. Arch Biochem Biophys. 1994 Jun;311(2):354-7. PMID:8203897
- ↑ Deconinck N, Messaaoui A, Ziereisen F, Kadhim H, Sznajer Y, Pelc K, Nassogne MC, Vanier MT, Dan B. Metachromatic leukodystrophy without arylsulfatase A deficiency: a new case of saposin-B deficiency. Eur J Paediatr Neurol. 2008 Jan;12(1):46-50. Epub 2007 Jul 5. PMID:17616409 doi:http://dx.doi.org/10.1016/j.ejpn.2007.05.004
- ↑ Matsuda J, Kido M, Tadano-Aritomi K, Ishizuka I, Tominaga K, Toida K, Takeda E, Suzuki K, Kuroda Y. Mutation in saposin D domain of sphingolipid activator protein gene causes urinary system defects and cerebellar Purkinje cell degeneration with accumulation of hydroxy fatty acid-containing ceramide in mouse. Hum Mol Genet. 2004 Nov 1;13(21):2709-23. Epub 2004 Sep 2. PMID:15345707 doi:http://dx.doi.org/10.1093/hmg/ddh281