We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Pheromone binding protein
From Proteopedia
(Difference between revisions)
| (11 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| + | <StructureSection load='3bfa' size='340' side='right' caption='Pheromone binding protein of honey bee complex with pheromone (PDB code [[3bfa]]).' scene=''> | ||
| + | __TOC__ | ||
==Introduction== | ==Introduction== | ||
| - | + | '''Pheromone binding proteins''' [http://en.wikipedia.org/wiki/Pheromone_binding_protein (PBP)] are type of Odorant binding proteins [http://en.wikipedia.org/wiki/Odorant-binding_protein (OBP)] - soluble proteins mediating the early stages of volatiles detection in both insects and vertebrates<ref>DOI:10.3389/fphys.2014.00320</ref>. The volatiles (pheromones and other small hydrophobic molecules) are solubilized by the OBPs and activate the chemoreceptors. | |
| - | + | ||
| - | <ref>DOI:10.3389/fphys.2014.00320</ref>. | + | |
| - | The volatiles (pheromones and other small hydrophobic molecules) are solubilized by the OBPs and activate the chemoreceptors. | + | |
As a model protein of this family we will further describe the well investigated Pheromone binding protein of the honey bee, ASP1. | As a model protein of this family we will further describe the well investigated Pheromone binding protein of the honey bee, ASP1. | ||
| Line 9: | Line 8: | ||
== Pheromone-binding protein ASP1 == | == Pheromone-binding protein ASP1 == | ||
| - | Chemical communication is crucial in social insects, where a complicated and delicate system of signals must be maintained in order to preserve the fragile equilibrium between the queen and the workers. | + | Chemical communication is crucial in social insects, where a complicated and delicate system of signals must be maintained in order to preserve the fragile equilibrium between the queen and the workers. In the hive of the honey bee [http://en.wikipedia.org/wiki/Apis_mellifera ''Apis mellifera''] this equilibrium exists partially due to the extraction of blend of substances called queen mandibular pheromone [http://en.wikipedia.org/wiki/Honey_bee_pheromones#Queen_mandibular_pheromone (QMP)], by the queen <ref>Winston, M.L., 1987. The Biology of the Honey Bee. Harvard University Press, Cambridge, MA.</ref>. The three major component of the QMP blend are: 9-keto-2(E)-decenoic acid (9-ODA) and 9-hydroxy-2(E)-decenoic acid (9-HDA R-(−) or S-(+)). |
| - | In the hive of the honey bee [http://en.wikipedia.org/wiki/Apis_mellifera ''Apis mellifera''] this equilibrium exists partially due to the extraction of blend of substances called queen mandibular pheromone [http://en.wikipedia.org/wiki/Honey_bee_pheromones#Queen_mandibular_pheromone (QMP)], by the queen <ref>Winston, M.L., 1987. The Biology of the Honey Bee. Harvard University Press, Cambridge, MA.</ref>. The three major component of the QMP blend are: 9-keto-2(E)-decenoic acid (9-ODA) and 9-hydroxy-2(E)-decenoic acid (9-HDA R-(−) or S-(+)). | + | Pheromone-binding protein [http://www.uniprot.org/uniprot/Q9U9J6/ ASP1] of the honeybee [http://en.wikipedia.org/wiki/Apis_mellifera ''Apis mellifera''] L. (Hymenoptera: Apidea) was first isolated and characterized by Danty ''et al''. (1998)<ref>DOI:10.1016/j.jmb.2008.04.048</ref> from the bee antennae. |
| - | Pheromone-binding protein [http://www.uniprot.org/uniprot/Q9U9J6/ | + | |
| - | + | ||
| - | + | ||
== Structure == | == Structure == | ||
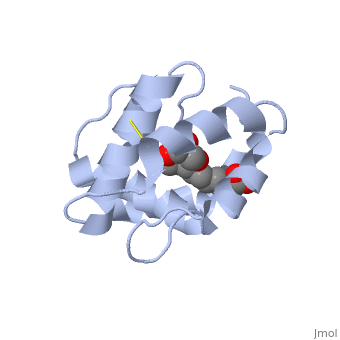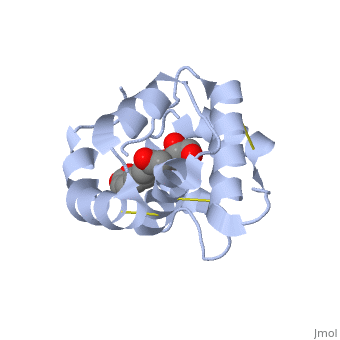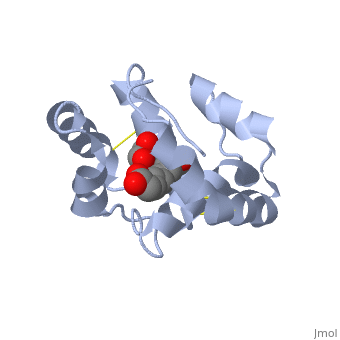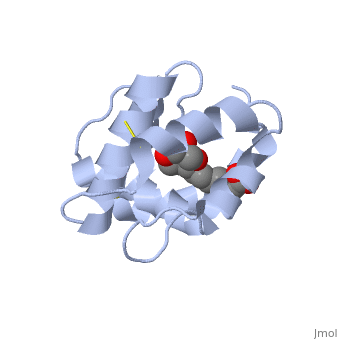
| - | The protein is composed of 144 amino acids, which forms 6 alpha helices. Three <scene name='60/609542/Disulfide_bonds/1'>3 disulfide bonds</scene> tied four helices: disulfide 20–51 between H1 and H3, 47– 98 between H3 and H6, and 107–89 between H6 and H5. | + | The protein is composed of 144 amino acids, which forms 6 alpha helices. Three <scene name='60/609542/Disulfide_bonds/1'>3 disulfide bonds</scene> formed by 6 Cystein residues tied four helices: disulfide 20–51 between H1 and H3, 47– 98 between H3 and H6, and 107–89 between H6 and H5. |
== Interaction with the ligand 9-ODA== | == Interaction with the ligand 9-ODA== | ||
| - | <scene name='60/609542/9-oda/3'>9-ODA</scene> | + | One of the main components of the QMP <scene name='60/609542/9-oda/3'>9-ODA</scene>, is binding to the protein binding site along with a <scene name='60/609542/Glycerol/2'>glycerol molecule</scene>.The carboxyl end of 9-ODA points towards the solvent, and has no bonds with residues of the protein. The residues in the binding site are <scene name='60/609542/Binding_site/3'>hydrophobic</scene>, and the connection between 9-ODA and ASP1 involve hydrogen bonds. |
| - | The carboxyl end of | + | |
| - | , and the connection between 9-ODA and ASP1 involve hydrogen bonds. | + | </StructureSection> |
| - | ---- | + | |
| + | ==3D structures of pheromone-binding protein== | ||
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| + | {{#tree:id=OrganizedByTopic|openlevels=0| | ||
| + | |||
| + | * Pheromone binding protein | ||
| + | |||
| + | **[[2h8v]], [[3bjh]], [[3cab]], [[3cdn]], [[3cz2]] – bPBP residues 26-144 – honey bee<br /> | ||
| + | **[[3d73]], [[3d74]], [[3d75]], [[3d76]], [[3d77]], [[3d78]] - bPBP residues 26-144 (mutant)<br /> | ||
| + | **[[1dqe]], [[2fjy]] – sPBP – silkworm<br /> | ||
| + | **[[1xfr]] – sPBP – NMR<br /> | ||
| + | **[[1gm0]] – sPBP (mutant) – NMR<br /> | ||
| + | **[[1qwv]], [[1two]], [[2jpo]], [[6um9]] – mPBP – moth - NMR<br /> | ||
| + | **[[6vq5]] – mPBP <br /> | ||
| + | **[[7uo6]] – PBP2 – corn borer - NMR<br /> | ||
| + | **[[7vw8]], [[7vw9]] – bwPBP1 - bollworm<br /> | ||
| - | + | * Pheromone binding protein complex | |
| + | **[[3bfa]], [[3bfb]], [[3bfh]], [[3cyz]] – bPBP residues 26-144 + pheromone<br /> | ||
| + | **[[3cz0]], [[3cz1]] - bPBP residues 26-144 + N-butyl benzene sulfonamide<br /> | ||
| + | **[[3fe6]], [[3fe8]], [[3fe9]] - bPBP residues 26-144 + methyldotetracontane<br /> | ||
| + | **[[2p70]] – sPBP + odorant<br /> | ||
| + | **[[2p71]] – sPBP + iodohexadecane<br /> | ||
| + | **[[4inw]], [[4inx]] – PBP + hexadecadienal – ''Amyelois transitella''<br /> | ||
| + | **[[7vwa]] – bwPBP1 + odorant <br /> | ||
| + | }} | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | [[Category: Topic Page]] | ||
Current revision
| |||||||||||
3D structures of pheromone-binding protein
Updated on 23-August-2023
References
- ↑ Pelosi P, Iovinella I, Felicioli A, Dani FR. Soluble proteins of chemical communication: an overview across arthropods. Front Physiol. 2014 Aug 27;5:320. doi: 10.3389/fphys.2014.00320. eCollection, 2014. PMID:25221516 doi:http://dx.doi.org/10.3389/fphys.2014.00320
- ↑ Winston, M.L., 1987. The Biology of the Honey Bee. Harvard University Press, Cambridge, MA.
- ↑ Pesenti ME, Spinelli S, Bezirard V, Briand L, Pernollet JC, Tegoni M, Cambillau C. Structural basis of the honey bee PBP pheromone and pH-induced conformational change. J Mol Biol. 2008 Jun 27;380(1):158-69. Epub 2008 Apr 27. PMID:18508083 doi:10.1016/j.jmb.2008.04.048