This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Raghad zoubi
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | ==Example page for α- | + | ==Example page for α-Amylase== |
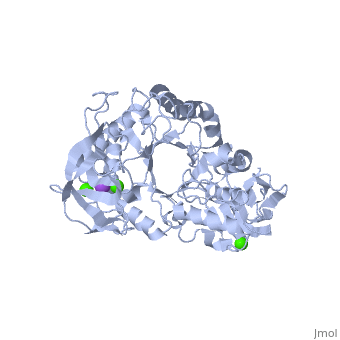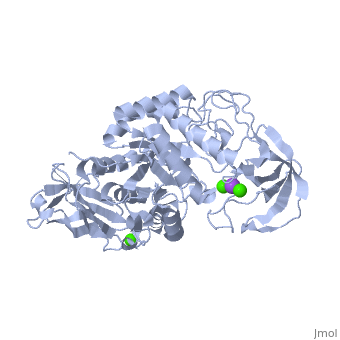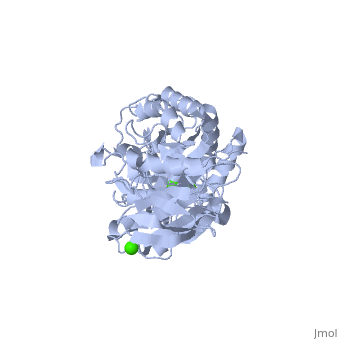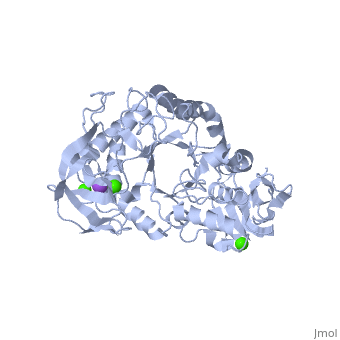
| - | <StructureSection load='1hvx' size='340' side='right' caption='α- | + | <StructureSection load='1hvx' size='340' side='right' caption='α-Amylase complex with Ca+2 (green) and Na+ (purple) ions (PDB code [[1hvx]])' scene=''> |
'''α-Amylase''' is a protein enzyme EC 3.2.1.1 that hydrolyses alpha bonds of large, alpha-linked polysaccharides, such as starch and glycogen, yielding glucose and maltose. It is the major form of amylase found in humans and other mammals.It is also present in seeds containing starch as a food reserve, and is secreted by many fungi. <ref>http://en.wikipedia.org/wiki/Alpha-amylase</ref> | '''α-Amylase''' is a protein enzyme EC 3.2.1.1 that hydrolyses alpha bonds of large, alpha-linked polysaccharides, such as starch and glycogen, yielding glucose and maltose. It is the major form of amylase found in humans and other mammals.It is also present in seeds containing starch as a food reserve, and is secreted by many fungi. <ref>http://en.wikipedia.org/wiki/Alpha-amylase</ref> | ||
| Line 10: | Line 10: | ||
The α-amylases form is also found in plants, fungi (ascomycetes and basidiomycetes) and bacteria (Bacillus) <ref>http://en.wikipedia.org/wiki/Amylase</ref> | The α-amylases form is also found in plants, fungi (ascomycetes and basidiomycetes) and bacteria (Bacillus) <ref>http://en.wikipedia.org/wiki/Amylase</ref> | ||
| - | ==Industrial use== | + | == Industrial use == |
α-Amylase is used in ethanol production to break starches in grains into fermentable sugars. | α-Amylase is used in ethanol production to break starches in grains into fermentable sugars. | ||
| Line 18: | Line 18: | ||
== Structural highlights == | == Structural highlights == | ||
| - | Shown as 1hvx is the structure of the thermostable α-amylase of Bacillus stearothermophilus (BSTA)[3]. BSTA is comprised of a single polypeptide chain. This chain is folded into three domains: A, B and C. These domains are generally found on all α-amylase enzymes. The <scene name='60/609816/A_domain/1'>A domain</scene> constitutes the core structure, with a (β/α)8-barrel.The <scene name='60/609816/B_domain/1'>B domain</scene> consists of a sheet of four anti-parallel β-strands with a pair of anti-parallel β-strands. Long loops are observed between the β-strands. Located within the B domain is the binding site for Ca2+-Na+-Ca2+.<scene name='60/609816/Domain_c/1'>Domain C</scene> consisting of eight β-strands is assembled into a globular unit forming a Greek key motif. It also holds the third Ca2+ binding site in association with domain A. Positioned on the C-terminal side of the β-strands of the (β/α)8-barrel in domain A is the active site. The catalytic residues involved for the BSTA active site are Asp234, Glu264, and Asp331 | + | Shown as 1hvx is the structure of the thermostable α-amylase of Bacillus stearothermophilus (BSTA)[3]. BSTA is comprised of a single polypeptide chain. This chain is folded into three domains: A, B and C. These domains are generally found on all α-amylase enzymes. The <scene name='60/609816/A_domain/1'>A domain</scene> constitutes the core structure, with a (β/α)8-barrel.The <scene name='60/609816/B_domain/1'>B domain</scene> consists of a sheet of four anti-parallel β-strands with a pair of anti-parallel β-strands. Long loops are observed between the β-strands. Located within the B domain is the binding site for Ca2+-Na+-Ca2+.<scene name='60/609816/Domain_c/1'>Domain C</scene> consisting of eight β-strands is assembled into a globular unit forming a Greek key motif. It also holds the third Ca2+ binding site in association with domain A. Positioned on the C-terminal side of the β-strands of the (β/α)8-barrel in domain A is the active site. The catalytic residues involved for the BSTA active site are<scene name='60/609816/Aga/1'> Asp234, Glu264, and Asp331</scene>.The residues are identical to other α-amylases, yet there are positional differences which reflect the flexible nature of catalytic resides. <scene name='60/609816/Ca1_ca2_na/1'>CaII and CaI with Na</scene> found in the interior of domain B and CaIII at the interface of domain A and C, constitute the metal ion binding sites. All α-amylases contain one strongly conserved Ca2+ ion for structural integrity and enzymatic activity.[4] CaI is consistent in α-amylases, however there are structural differences between the linear trio of CaI, CaII and Na in other enzymes. CaIII acts as a bridge between two loops, one from α6 of domain A, and one between β1 and β2 of domain C. |
</StructureSection> | </StructureSection> | ||
Current revision
Example page for α-Amylase
| |||||||||||
References
- ↑ http://en.wikipedia.org/wiki/Alpha-amylase
- ↑ http://en.wikipedia.org/wiki/Amylase
- ↑ "The use of enzymes in detergents". Faculty of Engineering, Science and the Built Environment, London South Bank University. 20 December 2004. Archived from the original on 20 October 2009. Retrieved 21 November 2009.