This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Neuroligin
From Proteopedia
(Difference between revisions)
| (19 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
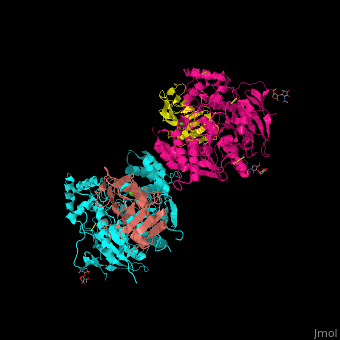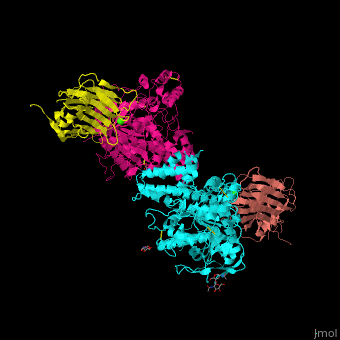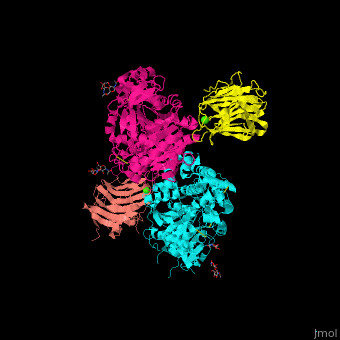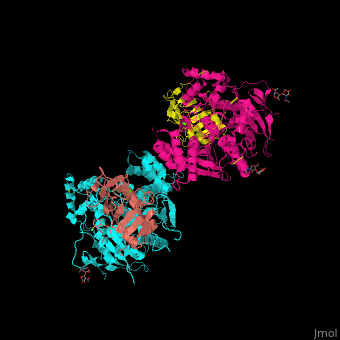
| - | + | <StructureSection load='' size='350' side='right' scene='42/422448/Cv/2' caption='Dimer of two mouse neuroligin-1 cholinesterase-like domains (magenta and cyan) in complex with two neurexin-beta1 LNS6 domains (salmon and yellow) and Ca+2 ions (green) [[3b3q]]'> | |
| - | + | ||
| + | == Function == | ||
| + | [[Neuroligin|Neuroligins]] (NLGN) are a family of postsynaptic proteins which bind to the presynaptic [[Neurexin]] (NRXN) to form a complex which attaches neurons. In humans the NLGN family contains 4 to 5 members that are numbered 1, 2, 3, 4X and 5 or 4Y<ref>PMID:18093521</ref>. | ||
| + | See [[Neurodevelopmental Disorders]]<br /> | ||
| + | [[Neuroligin-Neurexin Interaction]] | ||
| + | *'''NLGN-1''' regulates spines and synaptic plasticity<ref>PMID:26880202</ref>. <br /> | ||
| + | *'''NLGN-2''' is localized to inhibitory synapses<ref>PMID:15540461</ref>. <br /> | ||
| + | *'''NLGN-3''' is a synapse organizer which shapes normal function and autism disorder<ref>PMID:17897391</ref>. <br /> | ||
| + | *'''NLGN-4''' is localized to glyceric post-synapses and regulates retina inhibition<ref>PMID:21282647</ref>. <br /> | ||
| + | == Disease == | ||
| + | Mutations in NLGN 4 are associated with autism and mental retardation<ref>PMID:19726642</ref>. | ||
| - | + | == Structural insights == | |
| - | + | The <scene name='42/422448/Cv/5'>NLGN-NRXN complex involves the octahedrally coordinated Ca+2 ion</scene><ref>PMID:18084303</ref>. Water molecules are shown as red spheres. | |
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
== 3D Structures of Neuroligin == | == 3D Structures of Neuroligin == | ||
| Line 35: | Line 27: | ||
* Neuroligin | * Neuroligin | ||
| + | **[[5ojk]] - hNLGN-1 cholinesterase domain - human<br /> | ||
| + | **[[3bix]] - rNLGN-1 cholinesterase domain - rat<br /> | ||
| + | **[[8gs4]] - hNLGN-2 – Cryo EM<br /> | ||
**[[3bl8]] - mNLGN-2 cholinesterase domain – mouse<br /> | **[[3bl8]] - mNLGN-2 cholinesterase domain – mouse<br /> | ||
| + | **[[7cee]] - mNLGN-3 cholinesterase domain<br /> | ||
**[[3be8]] - hNLGN-4 cholinesterase domain – human<br /> | **[[3be8]] - hNLGN-4 cholinesterase domain – human<br /> | ||
| - | **[[3bix]] - rNLGN-1 cholinesterase domain - rat<br /> | ||
* Neuroligin+neurexin | * Neuroligin+neurexin | ||
| Line 45: | Line 40: | ||
**[[3biw]] - rNLGN-1 cholinesterase domain+NRXN-1β LNS domain<br /> | **[[3biw]] - rNLGN-1 cholinesterase domain+NRXN-1β LNS domain<br /> | ||
**[[3vkf]] - rNLGN-1 cholinesterase domain + bNRXN-1β LNS domain | **[[3vkf]] - rNLGN-1 cholinesterase domain + bNRXN-1β LNS domain | ||
| + | |||
| + | *NLGN other complexes | ||
| + | |||
| + | **[[5oj6]] - rNLGN-1 cholinesterase domain + MDGA1<br /> | ||
| + | **[[5v5v]] - rNLGN-2 cholinesterase domain + MDGA1<br /> | ||
| + | **[[5xeq]] - rNLGN-2 cholinesterase domain (mutant) + MDGA1<br /> | ||
| + | **[[7ceg]] - mNLGN-3 cholinesterase domain + PTP d<br /> | ||
| + | |||
}} | }} | ||
| + | == References == | ||
| + | <references/> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
3D Structures of Neuroligin
13-July-2023
References
- ↑ Fabrichny IP, Leone P, Sulzenbacher G, Comoletti D, Miller MT, Taylor P, Bourne Y, Marchot P. Structural analysis of the synaptic protein neuroligin and its beta-neurexin complex: determinants for folding and cell adhesion. Neuron. 2007 Dec 20;56(6):979-91. PMID:18093521 doi:10.1016/j.neuron.2007.11.013
- ↑ Liu A, Zhou Z, Dang R, Zhu Y, Qi J, He G, Leung C, Pak D, Jia Z, Xie W. Neuroligin 1 regulates spines and synaptic plasticity via LIMK1/cofilin-mediated actin reorganization. J Cell Biol. 2016 Feb 15;212(4):449-63. doi: 10.1083/jcb.201509023. PMID:26880202 doi:http://dx.doi.org/10.1083/jcb.201509023
- ↑ Varoqueaux F, Jamain S, Brose N. Neuroligin 2 is exclusively localized to inhibitory synapses. Eur J Cell Biol. 2004 Sep;83(9):449-56. PMID:15540461 doi:http://dx.doi.org/10.1078/0171-9335-00410
- ↑ Budreck EC, Scheiffele P. Neuroligin-3 is a neuronal adhesion protein at GABAergic and glutamatergic synapses. Eur J Neurosci. 2007 Oct;26(7):1738-48. doi: 10.1111/j.1460-9568.2007.05842.x. PMID:17897391 doi:http://dx.doi.org/10.1111/j.1460-9568.2007.05842.x
- ↑ Hoon M, Soykan T, Falkenburger B, Hammer M, Patrizi A, Schmidt KF, Sassoe-Pognetto M, Lowel S, Moser T, Taschenberger H, Brose N, Varoqueaux F. Neuroligin-4 is localized to glycinergic postsynapses and regulates inhibition in the retina. Proc Natl Acad Sci U S A. 2011 Feb 15;108(7):3053-8. doi:, 10.1073/pnas.1006946108. Epub 2011 Jan 31. PMID:21282647 doi:http://dx.doi.org/10.1073/pnas.1006946108
- ↑ Zhang C, Milunsky JM, Newton S, Ko J, Zhao G, Maher TA, Tager-Flusberg H, Bolliger MF, Carter AS, Boucard AA, Powell CM, Sudhof TC. A neuroligin-4 missense mutation associated with autism impairs neuroligin-4 folding and endoplasmic reticulum export. J Neurosci. 2009 Sep 2;29(35):10843-54. doi: 10.1523/JNEUROSCI.1248-09.2009. PMID:19726642 doi:http://dx.doi.org/10.1523/JNEUROSCI.1248-09.2009
- ↑ Chen X, Liu H, Shim AH, Focia PJ, He X. Structural basis for synaptic adhesion mediated by neuroligin-neurexin interactions. Nat Struct Mol Biol. 2008 Jan;15(1):50-6. Epub 2007 Dec 16. PMID:18084303 doi:10.1038/nsmb1350
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Alexander Berchansky, Joel L. Sussman, Pascale Marchot, Jaime Prilusky, David Canner