Hox protein
From Proteopedia
(Difference between revisions)
| (14 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load=' | + | {{BAMBED |
| + | |DATE=July 20, 2012 | ||
| + | |OLDID=1419976 | ||
| + | |BAMBEDDOI=10.1002/bmb.20650 | ||
| + | }} | ||
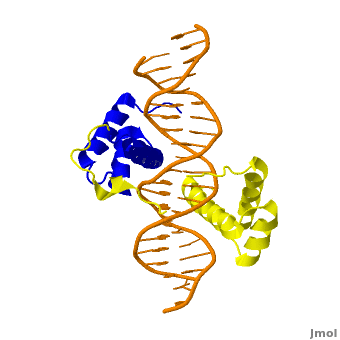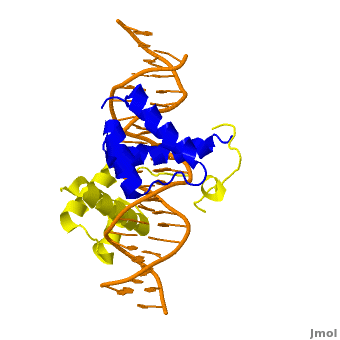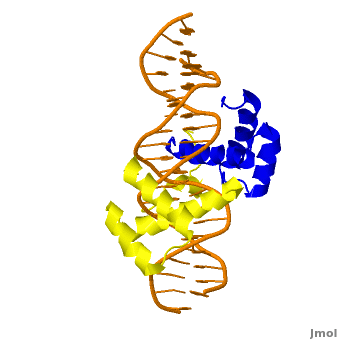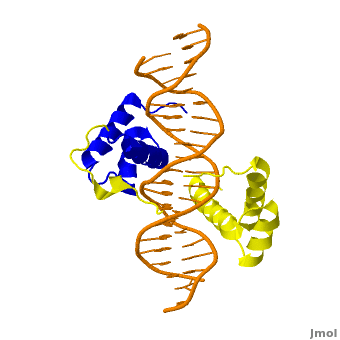
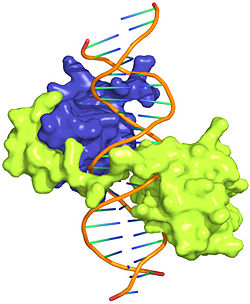
| + | <StructureSection load='' size='350' side='right' scene='Sandbox_Reserved_169/Complex/1' caption='Homeotic protein SEX complex with homeobox protein extradenticle and DNA (PDB code [[2r5z]]).'> | ||
''This is a joint project of students at La Cañada High School, La Cañada Flintridge, California USA, and students at the University of Southern California, Los Angeles, California USA, mentored by [[User:Remo Rohs|Professor Remo Rohs]].'' | ''This is a joint project of students at La Cañada High School, La Cañada Flintridge, California USA, and students at the University of Southern California, Los Angeles, California USA, mentored by [[User:Remo Rohs|Professor Remo Rohs]].'' | ||
| Line 9: | Line 14: | ||
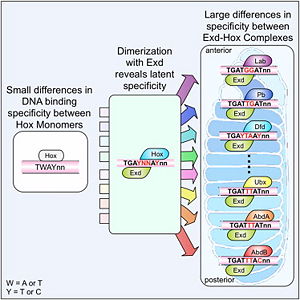
[[Image:Cell.jpg|thumb|right|300px|Figure 2: Hox proteins require a cofactor to achieve high binding specificity in order to execute their distinct functions in developing various parts of the fly embryo. Elsevier/Cell Press has provided permission for usage of this figure<ref name="slattery">Slattery M, Riley T, Liu P, Abe N, Gomez-Alcala P, Dror I, Zhou T, Rohs R, Honig B, Bussemaker HJ, Mann RS. Cofactor binding evokes latent differences in DNA binding specificity between Hox proteins. Cell. 2011;147(6):1270-82. [http://www.ncbi.nlm.nih.gov/pubmed/22153072 PMID:22153072]</ref>.]] | [[Image:Cell.jpg|thumb|right|300px|Figure 2: Hox proteins require a cofactor to achieve high binding specificity in order to execute their distinct functions in developing various parts of the fly embryo. Elsevier/Cell Press has provided permission for usage of this figure<ref name="slattery">Slattery M, Riley T, Liu P, Abe N, Gomez-Alcala P, Dror I, Zhou T, Rohs R, Honig B, Bussemaker HJ, Mann RS. Cofactor binding evokes latent differences in DNA binding specificity between Hox proteins. Cell. 2011;147(6):1270-82. [http://www.ncbi.nlm.nih.gov/pubmed/22153072 PMID:22153072]</ref>.]] | ||
| - | + | {{Clear}} | |
| - | Hox proteins are transcription factors that play a key role in the '''embryonic development''' across species by activating and repressing genes. In ''Drosophila,'' eight Hox proteins are responsible for the development of different body segments of the fly, such as its antennae, wings, or legs. Hox proteins execute their distinct functions through binding to similar but different in vivo binding sites<ref>Mann RS, Lelli KM, Joshi R. Hox specificity unique roles for cofactors and collaborators. Curr Top Dev Biol. 2009;88:63-101. [http://www.ncbi.nlm.nih.gov/pubmed/19651302 PMID:19651302]</ref>. This page discusses molecular mechanisms through which Hox proteins recognize their DNA targets with very high binding specificity. <br/> | + | '''Hox proteins''' or '''homeobox proteins''' are transcription factors that play a key role in the '''embryonic development''' across species by activating and repressing genes. In ''Drosophila,'' eight Hox proteins are responsible for the development of different body segments of the fly, such as its antennae, wings, or legs. Hox proteins execute their distinct functions through binding to similar but different in vivo binding sites<ref>Mann RS, Lelli KM, Joshi R. Hox specificity unique roles for cofactors and collaborators. Curr Top Dev Biol. 2009;88:63-101. [http://www.ncbi.nlm.nih.gov/pubmed/19651302 PMID:19651302]</ref>. This page discusses molecular mechanisms through which Hox proteins recognize their DNA targets with very high binding specificity. <br/> |
The crystal structure of a Hox-DNA complex (Figure 1) shows that the Hox protein ''Sex combs reduced'' (Scr) binds its specific ''in vivo'' site with the help of cofactors, ''Extradenticle (Exd)/Pbx proteins''. Hox proteins can bind DNA as monomers but their binding specificity is enhanced when the co-factor is present, a principle that is called '''latent specificity''' (Figure 2). In ''Drosophila'', for instance, eight Hox proteins bind as heterodimers with their cofactor Exd to similar but distinct target sites.<br/> | The crystal structure of a Hox-DNA complex (Figure 1) shows that the Hox protein ''Sex combs reduced'' (Scr) binds its specific ''in vivo'' site with the help of cofactors, ''Extradenticle (Exd)/Pbx proteins''. Hox proteins can bind DNA as monomers but their binding specificity is enhanced when the co-factor is present, a principle that is called '''latent specificity''' (Figure 2). In ''Drosophila'', for instance, eight Hox proteins bind as heterodimers with their cofactor Exd to similar but distinct target sites.<br/> | ||
| Line 44: | Line 49: | ||
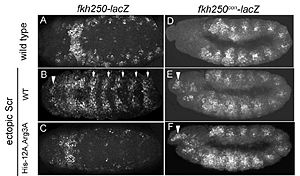
[[Image:Joshi-etal-Figure7.jpg |thumb|left|300px|Figure 4: Expression patterns of Scr in presence of Scr specific site (left panel) vs. Hox consensus site (right panel). Elsevier/Cell Press has provided permission for usage of this figure<ref name="joshi"/>.]] | [[Image:Joshi-etal-Figure7.jpg |thumb|left|300px|Figure 4: Expression patterns of Scr in presence of Scr specific site (left panel) vs. Hox consensus site (right panel). Elsevier/Cell Press has provided permission for usage of this figure<ref name="joshi"/>.]] | ||
| - | + | {{Clear}} | |
In vitro binding studies have shown that His-12 and Arg-3 mutations have a large effect when exposed to the Scr specific site, whereas the effect is small when exposed to a Hox consensus site. The biological importance of both side chains becomes apparent in ''in vivo'' experiments. Upon mutations of His-12 and Arg-3 to alanine, Scr expression in a fly embryo is dramatically affected (Figure 4). In comparison to wild type Scr (A) and based on ectopic expression (B), there is only residual expression detected in | In vitro binding studies have shown that His-12 and Arg-3 mutations have a large effect when exposed to the Scr specific site, whereas the effect is small when exposed to a Hox consensus site. The biological importance of both side chains becomes apparent in ''in vivo'' experiments. Upon mutations of His-12 and Arg-3 to alanine, Scr expression in a fly embryo is dramatically affected (Figure 4). In comparison to wild type Scr (A) and based on ectopic expression (B), there is only residual expression detected in | ||
the thorax region of the double mutant when the Scr specific site is tested (C), whereas there is no apparent effect on expression in the presence of a Hox consensus site (D-F).<br/> | the thorax region of the double mutant when the Scr specific site is tested (C), whereas there is no apparent effect on expression in the presence of a Hox consensus site (D-F).<br/> | ||
| Line 54: | Line 59: | ||
[[Image:Cell2007-Fig4.jpg |thumb|left|300px|Figure 6: Comparison of DNA shape of Scr specific in vivo site (left panel) vs. Hox consensus site (right panel). Elsevier/Cell Press has provided permission for usage of this figure<ref name="joshi"/>.]] | [[Image:Cell2007-Fig4.jpg |thumb|left|300px|Figure 6: Comparison of DNA shape of Scr specific in vivo site (left panel) vs. Hox consensus site (right panel). Elsevier/Cell Press has provided permission for usage of this figure<ref name="joshi"/>.]] | ||
| - | + | {{Clear}} | |
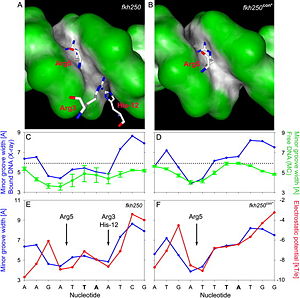
Based on the comparison of the two crystal structures of a Scr-Exd-DNA ternary complexes (Figure 6), it was found that three N-terminal residues contact the minor groove of the Scr specific site ''fkh250'' (A) compared to only Arg5 binding the Hox consensus site ''fkh250con'' (B). In their protein-bound states, the shapes of both sites are distinct (dark gray, concave; green, convex surfaces). The distinct shapes of the two DNA binding sites, shown as minor groove width in the crystal structures of the complexes (blue plots), are already present when the protein is not bound to the DNA, with two minima in ''fkh250'' (C) vs. one minimum in ''fkh250con'' (D), as inferred by Monte Carlo simulations (green plots). Minor groove width (blue plots) and electrostatic potential (red plots) correlate and form two binding pockets in ''fkh250'' (E) and only a binding site for Arg5 in ''fkh250con'' (F).<br/> | Based on the comparison of the two crystal structures of a Scr-Exd-DNA ternary complexes (Figure 6), it was found that three N-terminal residues contact the minor groove of the Scr specific site ''fkh250'' (A) compared to only Arg5 binding the Hox consensus site ''fkh250con'' (B). In their protein-bound states, the shapes of both sites are distinct (dark gray, concave; green, convex surfaces). The distinct shapes of the two DNA binding sites, shown as minor groove width in the crystal structures of the complexes (blue plots), are already present when the protein is not bound to the DNA, with two minima in ''fkh250'' (C) vs. one minimum in ''fkh250con'' (D), as inferred by Monte Carlo simulations (green plots). Minor groove width (blue plots) and electrostatic potential (red plots) correlate and form two binding pockets in ''fkh250'' (E) and only a binding site for Arg5 in ''fkh250con'' (F).<br/> | ||
==High-throughput Analysis of Hox-DNA Binding Specificity== | ==High-throughput Analysis of Hox-DNA Binding Specificity== | ||
| - | [[Image:Slattery-etal-Figure6.jpg |thumb|right|300px|Figure 7: DNA shape | + | [[Image:Slattery-etal-Figure6.jpg |thumb|right|300px|Figure 7: DNA shape |
| - | + | ==3D structure of Hox protein== | |
| + | Updated on {{REVISIONDAY2}}-{{MONTHNAME|{{REVISIONMONTH}}}}-{{REVISIONYEAR}} | ||
| - | + | [[1b72]] – hHox-B1 + DNA – human<br /> | |
| + | [[1puf]] – hHox-PRL + mHox-A9 + DNA <br /> | ||
| + | [[2l7z]] – hHox-A13 + DNA – NMR<br /> | ||
| + | [[2lp0]] – hHox-A13 + geminin peptide – NMR<br /> | ||
| + | [[2cra]] – hHox-B13 – NMR<br /> | ||
| + | [[5edn]], [[5eea]], [[5ef6]], [[5no6]] – hHox-B13 + DNA<br /> | ||
| + | [[5eg0]], [[5ego]] – hHox-B13 + MEISI1 + DNA<br /> | ||
| + | [[2msy]] – hHox-C9 – NMR<br /> | ||
| + | [[3a03]] – hHox-11L1<br /> | ||
| + | [[1ig7]] – mHox-MSX + DNA – mouse<br /> | ||
| + | [[1lfu]] – mHox-PBX + DNA – NMR<br /> | ||
| + | [[2ld5]] – mHox-A13 + DNA – NMR<br /> | ||
| + | [[4uut]] – DmHox – ''Drosophila melanogaster''<br /> | ||
| + | [[2r5z]] – DmHox + DNA <br /> | ||
| + | [[2r5y]] – DmHox + Scr + DNA <br /> | ||
| + | [[4uus]], [[5cyc]] – DmHox + Ubx+ DNA <br /> | ||
| + | [[5zjq]], [[5zjr]], [[5zjs]], [[5zjt]] – DmHox + AbdB+ DNA <br /> | ||
| - | + | == References == | |
| - | + | <references/> | |
| - | + | ||
| - | + | ||
| - | + | [[Category:Featured in BAMBED]] | |
| - | + | [[Category:Topic Page]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
This page, as it appeared on July 20, 2012, was featured in this article in the journal Biochemistry and Molecular Biology Education.
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
Remo Rohs, Eric Martz, Michal Harel, Joel L. Sussman, Skyler Saleebyan, Julia Tam, Bailey Holmes, Sharon Kim, Alexander Berchansky, Iris Dror, Ana Carolina Dantas Machado, Masha Karelina, Keziah Kim, Jaime Prilusky, Angel Herraez