Molecular Playground/CsoR and RcnR
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
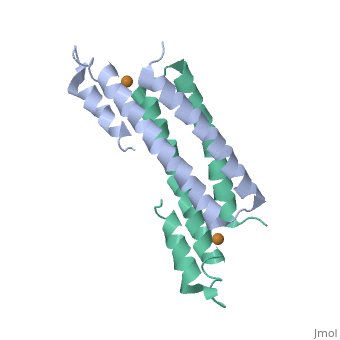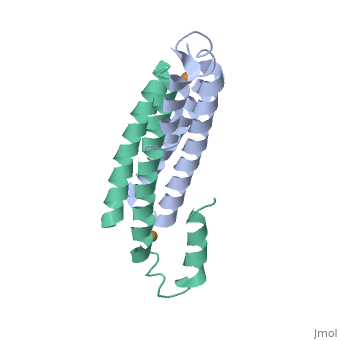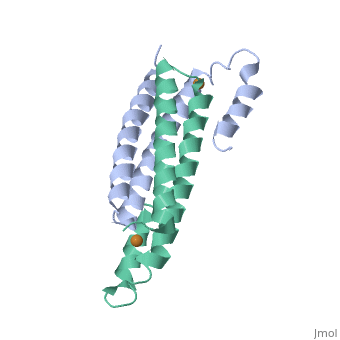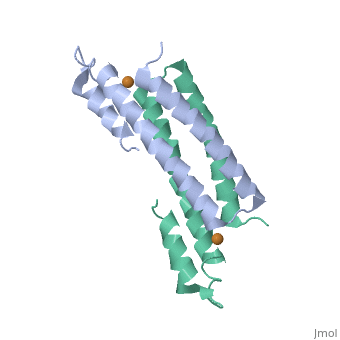
| - | + | <StructureSection load='2hh7' size='350' side='right' caption='Cu(I)-bound CsoR (PDB ID: [http://www.rcsb.org/pdb/explore/explore.do?structureId=2HH7 2HH7])' scene=''> | |
| - | < | + | |
| - | caption='Cu(I)-bound CsoR (PDB ID: [http://www.rcsb.org/pdb/explore/explore.do?structureId=2HH7 2HH7])' | + | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
One of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst and on display at the [http://www.molecularplayground.org/ Molecular Playground]. | One of the [[CBI Molecules]] being studied in the [http://www.umass.edu/cbi/ University of Massachusetts Amherst Chemistry-Biology Interface Program] at UMass Amherst and on display at the [http://www.molecularplayground.org/ Molecular Playground]. | ||
| Line 75: | Line 23: | ||
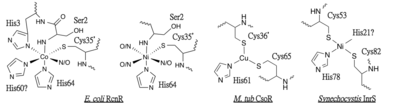
InrS conserves all the Cu(I) binding residues identified in [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''M. tuberculosis''] CsoR, Cys53, Cys82 and His78, which have been shown as Ni(II) ligands. His21 is also important for metal binding, while it remains unclear whether or not His21 is a ligand involved in this square planar metal site. Added to this, InrS also lacks the second coordination sphere hydrogen bond network.<ref>PMID: 24666373</ref> | InrS conserves all the Cu(I) binding residues identified in [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis ''M. tuberculosis''] CsoR, Cys53, Cys82 and His78, which have been shown as Ni(II) ligands. His21 is also important for metal binding, while it remains unclear whether or not His21 is a ligand involved in this square planar metal site. Added to this, InrS also lacks the second coordination sphere hydrogen bond network.<ref>PMID: 24666373</ref> | ||
| - | [[Image:CBI metal site.png| | + | [[Image:CBI metal site.png|400px|left|thumb|Fig. 1: Schematic of known Ni(II) and Co(II) binding residues in the metal sites of RcnR, Cu(I) binding residues in CsoR and Ni(II) binding site in InrS. Figure made with ChemDraw.]] |
| Line 95: | Line 43: | ||
The mechanism of DNA binding of the CsoR/RcnR family of metal-responsive transcriptional regulators is still unknown. Additionally, RcnR has an added level of complexity because it is reponsive to both Ni(II) and Co(II) binding. The [http://people.chem.umass.edu/mmaroney/ Maroney Lab] at the University of Massachusetts Amherst is interested in the conformational changes of RcnR induced by DNA-, Ni(II)-, and Co(II)-binding. Identification of the remaining metal binding residues in RcnR is ongoing, in addition to identification of the DNA-binding residues in RcnR, as there is no crystal structure of any member of this family of proteins binding to DNA. | The mechanism of DNA binding of the CsoR/RcnR family of metal-responsive transcriptional regulators is still unknown. Additionally, RcnR has an added level of complexity because it is reponsive to both Ni(II) and Co(II) binding. The [http://people.chem.umass.edu/mmaroney/ Maroney Lab] at the University of Massachusetts Amherst is interested in the conformational changes of RcnR induced by DNA-, Ni(II)-, and Co(II)-binding. Identification of the remaining metal binding residues in RcnR is ongoing, in addition to identification of the DNA-binding residues in RcnR, as there is no crystal structure of any member of this family of proteins binding to DNA. | ||
| - | + | </StructureSection> | |
==3D structures of copper homeostasis protein== | ==3D structures of copper homeostasis protein== | ||
Current revision
| |||||||||||
3D structures of copper homeostasis protein
References
- ↑ Reyes-Caballero H, Campanello GC, Giedroc DP. Metalloregulatory proteins: metal selectivity and allosteric switching. Biophys Chem. 2011 Jul;156(2-3):103-14. Epub 2011 Apr 5. PMID:21511390 doi:10.1016/j.bpc.2011.03.010
- ↑ Foster AW, Patterson CJ, Pernil R, Hess CR, Robinson NJ. Cytosolic Ni(II) sensor in cyanobacterium: nickel detection follows nickel affinity across four families of metal sensors. J Biol Chem. 2012 Apr 6;287(15):12142-51. doi: 10.1074/jbc.M111.338301. Epub 2012, Feb 22. PMID:22356910 doi:http://dx.doi.org/10.1074/jbc.M111.338301
- ↑ Iwig JS, Chivers PT. Coordinating intracellular nickel-metal-site structure-function relationships and the NikR and RcnR repressors. Nat Prod Rep. 2010 May;27(5):658-67. Epub 2010 Mar 5. PMID:20442957 doi:10.1039/b906683g
- ↑ Smaldone GT, Helmann JD. CsoR regulates the copper efflux operon copZA in Bacillus subtilis. Microbiology. 2007 Dec;153(Pt 12):4123-8. PMID:18048925 doi:10.1099/mic.0.2007/011742-0
- ↑ Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007 Jan;3(1):60-8. Epub 2006 Dec 3. PMID:17143269 doi:http://dx.doi.org/10.1038/nchembio844
- ↑ Foster AW, Patterson CJ, Pernil R, Hess CR, Robinson NJ. Cytosolic Ni(II) sensor in cyanobacterium: nickel detection follows nickel affinity across four families of metal sensors. J Biol Chem. 2012 Apr 6;287(15):12142-51. doi: 10.1074/jbc.M111.338301. Epub 2012, Feb 22. PMID:22356910 doi:http://dx.doi.org/10.1074/jbc.M111.338301
- ↑ Iwig JS, Leitch S, Herbst RW, Maroney MJ, Chivers PT. Ni(II) and Co(II) sensing by Escherichia coli RcnR. J Am Chem Soc. 2008 Jun 18;130(24):7592-606. Epub 2008 May 28. PMID:18505253 doi:10.1021/ja710067d
- ↑ Liu T, Ramesh A, Ma Z, Ward SK, Zhang L, George GN, Talaat AM, Sacchettini JC, Giedroc DP. CsoR is a novel Mycobacterium tuberculosis copper-sensing transcriptional regulator. Nat Chem Biol. 2007 Jan;3(1):60-8. Epub 2006 Dec 3. PMID:17143269 doi:http://dx.doi.org/10.1038/nchembio844
- ↑ Higgins KA, Hu HQ, Chivers PT, Maroney MJ. Effects of select histidine to cysteine mutations on transcriptional regulation by Escherichia coli RcnR. Biochemistry. 2013 Jan 8;52(1):84-97. doi: 10.1021/bi300886q. Epub 2012 Dec 24. PMID:23215580 doi:http://dx.doi.org/10.1021/bi300886q
- ↑ Higgins KA, Chivers PT, Maroney MJ. Role of the N-terminus in determining metal-specific responses in the E. coli Ni- and Co-responsive metalloregulator, RcnR. J Am Chem Soc. 2012 Apr 25;134(16):7081-93. doi: 10.1021/ja300834b. Epub 2012 Apr, 11. PMID:22471551 doi:http://dx.doi.org/10.1021/ja300834b
- ↑ Foster AW, Pernil R, Patterson CJ, Robinson NJ. Metal specificity of cyanobacterial nickel-responsive repressor InrS: cells maintain zinc and copper below the detection threshold for InrS. Mol Microbiol. 2014 May;92(4):797-812. doi: 10.1111/mmi.12594. Epub 2014 Apr 14. PMID:24666373 doi:http://dx.doi.org/10.1111/mmi.12594
Also See
Proteopedia Page Contributors and Editors (what is this?)
Carolyn Carr, Hsin-Ting Huang, Heidi Hu, Alexander Berchansky, Michal Harel