This old version of Proteopedia is provided for student assignments while the new version is undergoing repairs. Content and edits done in this old version of Proteopedia after March 1, 2026 will eventually be lost when it is retired in about June of 2026.
Apply for new accounts at the new Proteopedia. Your logins will work in both the old and new versions.
Sandbox Reserved 960
From Proteopedia
(Difference between revisions)
| (137 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
{{Sandbox_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | {{Sandbox_ESBS}}<!-- PLEASE ADD YOUR CONTENT BELOW HERE --> | ||
| - | = | + | =Antennal Specific Protein-1 from ''Apis mellifera'' (AmelASP1) with a serendipitous ligand at pH 5.5= |
| - | <StructureSection load='3fe6' size=' | + | <StructureSection load='3fe6' size='400' side='right' |
This is a default text for your page ''''''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | This is a default text for your page ''''''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| - | + | <nowiki> | |
| - | + | The protein '''AmelASP1''' has been identified in the antennae from the honeybee ''Apis mellifera''. Its primary sequence is a 144 amino acids polypeptide with a molecular weight of 13.180 kDa. AmelASP1 is part of the '''Pheromone Binding Protein (PBP)''' family. The 3D representation shown below was obtained at pH 5.5 using the [http://www-dsv.cea.fr/en/life-science-div/all-the-news/scientific-results/nanodrops-for-bioactive-compound-synthesis-and-screening nano-drops technique]. | |
| - | == Disease == | ||
| - | == | + | ==Biological function== |
| + | As many other social insects, honeybees employ a large varsity of pheromones to ensure intraspecific communication in several behavioral contexts. | ||
| + | The social organization of the hive is strongly determined by chemical signals, also known as pheromones, that are actively produced and transmitted by the queen. | ||
| + | |||
| + | ===Social relevance=== | ||
| + | The diets of workers bees and queen bee are strongly different and determinate diverse behaviors. | ||
| + | Workers bees are fed with royal jelly for only three days after egg-laying whereas the queen bee eats royal jelly during her whole life. She controls the activity of each bees by chemical communication. | ||
| + | Actually, the queen bee is the only one able to produce [http://www.chemspider.com/Chemical-Structure.1362276.html 9-ODA] - the main component of its pheromone which induces sexual or endocrine responses. This substance is sent to the workers bees which detect it through pheromone-binding proteins (PBPs). It is then transformed in [http://www.chemspider.com/Chemical-Structure.4472227.html 9-HDA] and added in the royal jelly. This last substance is eaten by the queen. In turn, queen bee transforms 9-HDA into 9-ODA. | ||
| + | Thus, ASP1 is primordial to the internal pheromone’s transport cycle in the hive. By binding the component of queen bee pheromone, bees express and transmit essential behaviour within the hive. Indeed, 9-ODA is responsible, among others, of preventing workers bees’ ovarian development. | ||
| + | |||
| + | ===Location in the antenna and transport of pheromones=== | ||
| + | ASP1 is a protein which is only produced in the antenna of drones and workers bees. This organ constitutes one major component of the bees’ olfactory system. Cuticules structures of these antennas shelter sensillae which are a gate for pheromones. These sensillae contain neurons. Branched endings are surrounded with sensillar lymph where ASP1 captures 9-ODA and transports it to pheromone receptor in the neuron membrane. PBP’s function is to solubilize {{Template:ColorKey_Hydrophobic}} odorant molecules, prevent their degradation and to transport them to reach the olfactory receptor. | ||
| + | |||
| + | |||
| + | == Structure == | ||
| + | === Domains and family === | ||
| + | The <scene name='60/604479/C-term/1'>C-terminal</scene> domain of this molecule presents a characteristic PBP-GOP domain. While this protein is composed of 144 residues the PBP domain begins at the 25th residue. | ||
| + | |||
| + | === Key residues === | ||
| + | AmelASP1 is composed of <scene name='60/604479/Helixes/1'>7 right-handed alpha helices</scene><ref> http://www.genome.jp/dbget-bin/www_bget?pdb:3FE6</ref> | ||
| + | ** <scene name='60/604479/H1/2'>H1</scene>: residues 8–25 | ||
| + | ** <scene name='60/604479/H2/2'>H2</scene>: residues 27–36 | ||
| + | ** <scene name='60/604479/H3/2'>H3</scene>: residues 42–56 | ||
| + | ** <scene name='60/604479/H4/1'>H4</scene>: residues 66–74 | ||
| + | ** <scene name='60/604479/H5/1'>H5</scene>: residues 75–77 (rarely mentioned in publications because of its tiny size) | ||
| + | ** <scene name='60/604479/H6/1'>H6</scene>: residues 78–90 | ||
| + | ** <scene name='60/604479/H7/1'>H7</scene>: residues 96–112 | ||
| + | |||
| + | <scene name='60/604479/H1/2'>H1</scene> has a break in the hydrogen-bonding pattern of its structure, forming tight substitute hydrogen bonds with water molecules. Thus, it results in a <scene name='60/604479/Kink/1'>kink</scene> (at residue Ala 14) induced by a disruption in the helical conformation, due to hydrogen bonds with water molecules.<ref>PMID: 18508083</ref> | ||
| + | |||
| + | === Components implicated in the structure rigidity === | ||
| + | AmelASP1 presents <scene name='60/604479/Disulfide_bonds/1'> three disulfide bridges</scene> which are greatly enhancing its structure’s rigidity by linking four of the helices together. The six cysteins and their interval spacing are the most striking features shared by proteins belonging to the PBP family. | ||
| + | |||
| + | The <scene name='60/604479/1st_disulfide_bridge/1'>first disulfide bridge</scene> is established between <scene name='60/604479/H1/2'>H1</scene> and <scene name='60/604479/H3/2'>H3</scene> through Cysteines 20 and 51. <scene name='60/604479/2nd_disulfide_bridge/2'>An other disulfide bridge</scene> links <scene name='60/604479/H3/2'>H3</scene> and <scene name='60/604479/H7/1'>H7</scene> through Cys 47 and 98, and the <scene name='60/604479/3rd_disulfide_bridge/1'>third disulfide bridge</scene> connects <scene name='60/604479/H6/1'>H6</scene> and <scene name='60/604479/H7/1'>H7</scene> thanks to Cys 89 and Cys 107. | ||
| + | |||
| + | Furthermore, non covalent bonds also play an important role. | ||
| + | Indeed, at pH 5.5, among the numerous other, two hydrogen bonds are particularly noticeable because of their importance in the formation of the loop stabilizing <scene name='60/604479/H4/1'>H4</scene>. <scene name='60/604479/Hydrogene_bond_1/2'>The first bond</scene> is established by Asp 66 and Leu 58 whereas <scene name='60/604479/Hydrogene_bond_2/1'>the second</scene> is formed between Asp 60 and Ala 63. | ||
| + | |||
| + | === Cavity === | ||
| + | The dynamic structure of the protein is responsible of the ligand’s binding by adjustment of position. The successful delivery of the effector to the receptor relies on this property. The ligand binding pocket consists in a <scene name='60/604479/Cavity/3'>cavity</scene> <ref>PMID: 25337796</ref> formed by the helices H2, H3, H4, H5 and H6 arranged in a globular shape which leads to a clear separation of the ligand from the {{Template:ColorKey_Polar}} environment. | ||
| + | The top of the cavity is not closed and can establish contacts with the solvent. The cavity is prone to accept ligand such as 9-ODA because of its specific composition. Indeed, cavity's components are mainly <scene name='60/604479/Hydrophobic_residues/2'>hydrophobic and aromatic</scene> <ref>PMID: 14594955</ref>.They consequently interact with the ligand's {{Template:ColorKey_Hydrophobic}} carbon chain and are localized on the internal face of the helix.Thus, it implies that these residues respect a regular distance pattern in the primary structure of the AmelASP1. | ||
| + | |||
| + | === pH influence === | ||
| + | pH affects the flexibility of ASP1 because it induces a different protonation state of the <scene name='60/604479/Ionizable_residues/1'>ionizable residues</scene>. Protonated residues give rise to micro-environnment changes which propagate all along the protein. Consequently, ASP1 is no longer able to interact with its ligands even if ionizable residues are distant from the cavity. <ref>PMID: 19481550</ref> | ||
| + | In fact, depending of the pH level, Asp35 bend the C terminal domain against the cavity. | ||
| + | At pH 5.5, <scene name='60/604479/C-term_asp35/1'>Asp 35</scene> is protonated and C terminal domain isn’t bend against the cavity. While ASP1 is a monomere at acid pH, it can dimerize at neutral and basic pH.<ref>PMID: 25337796</ref> | ||
| + | |||
| + | |||
| + | == Ligands == | ||
| + | [[Image:CMJ_Ligplot.png|150px|right|thumb|'''Fig.1''' CMJ Ligplot<ref>http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=3fe6&template=ligands.html&l=1.1</ref>]] | ||
| + | In order to determine this {{Template:ColorKey Composition Protein}}’s structure, several {{Template:ColorKey Composition Ligand}} has been used at pH 5.5 because this low pH fits with its natural medium in the bee antenna. | ||
| + | |||
| + | The three ligands used to characterize and purify AmelASP1 are : | ||
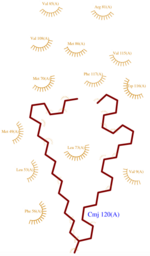
| + | *<scene name='60/604479/Cmj/3'>CMJ</scene> also known as (20s)-20-Methyldotetracontane, is a serendipitous ligand. This term signify that the purification of this molecule was completely fortuitous. It is a big unsaturated mono-methyl branched carbone chain with formula C43H88. This ligand fits in the {{Template:ColorKey_Hydrophobic}} cavity of AmelASP1 thanks to several interactions with <scene name='60/604479/Cmj_binding_residues/6'>specific residues.</scene> ('''Fig.1''') | ||
| + | |||
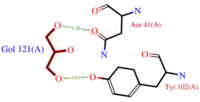
| + | *[[Image:GOL_Ligplot.png|200px|left|thumb|'''Fig.2''' GOL Ligplot<ref>http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=3fe6&template=ligands.html&l=2.1</ref>]]<scene name='60/604479/Gol/1'>Glycerol</scene> (C3H8O3) also known as GOL, is a ligand used for cryoprotection during the purification process of the protein. It is supposedly helping the main ligand to reach its binding site. | ||
| + | To do so, GOL links to<scene name='60/604479/Gol_binding_residues/1'> Asn 41 and Tyr 102.</scene> ('''Fig.2''') | ||
| + | |||
| + | [[Image:Cl_Ligplot.png|right|thumb|'''Fig.3''' Cl Ligplot<ref>http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=3fe6&template=ligands.html&o=METAL&l=1.1</ref>]] | ||
| + | |||
| + | |||
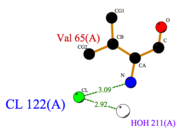
| + | *<scene name='60/604479/Cl/1'>Chloride ion</scene> facilitates the binding of other ligands to the protein. Its abundance around ASP1 varies according to changing pH conditions. At pH 5.5, <scene name='60/604479/Cl_binding_residue/1'>Val 65</scene> is here the only amino acid able to fix a chloride ion. ('''Fig.3''') | ||
| + | |||
| + | However, in natural conditions, binding ligands are the pheromones secreted by the queen such as 9-ODA. | ||
| + | |||
| + | |||
| + | == Related structures == | ||
| + | The structures shown below representing AmelASP1 with variable ligands and pH, emphasize and illustrate the binding versatility of Pheromones Binding Proteins. | ||
| + | The same PBP and their ligands can be crystalized either in apo (without ligand) or holo states (with ligand). | ||
| + | *In the apo state, the C terminus part of the protein obstructs the binding site. | ||
| + | **[[3cdn]] The same protein in apo form soaked at pH 4.0 <br /> | ||
| + | **[[2h8v]] The same protein in apo form at pH 5.5 <br /> | ||
| + | **[[3cz2]] The same protein in apo form at pH 7.0 <br /> | ||
| + | |||
| + | *In the holo state, depending of the ligand, the volume of the cavity changes. Likewise, presence of ligand and favorable pH, induce C terminus conformation changes. | ||
| + | **[[3fe8]] The same protein in complex with a serendipitous ligand soaked at pH 4.0 <br /> | ||
| + | **[[3fe9]] The same protein in complex with a serendipitous ligand soaked at pH 7.0 <br /> | ||
| + | **[[3bfa]] The same protein in complex with the QMP at pH 5.5 <br /> | ||
| + | **[[3bfb]] The same protein in complex with the 9-ODA at pH 5.5 <br /> | ||
| + | **[[3bfh]] The same protein in complex with the HDOA at pH 5.5 <br /> | ||
| + | **[[3bjh]] The same protein in complex with the nBBSA at pH 5.5 <br /> | ||
| + | **[[3cyz]] The same protein in complex with the 9-ODA at pH 7.0 <br /> | ||
| + | **[[3cz0]] The same protein in complex with the QMP at pH 7.0 <br /> | ||
| + | **[[3cz1]] The same protein in complex with the nBBSA at pH 7.0 <br /> | ||
| + | **[[3cab]] The same protein in complex with the nBBSA soaked at pH 7.0 <br /> | ||
| - | == Structural highlights == | ||
| - | This is a sample scene created with SAT to <scene name="/12/3456/Sample/1">color</scene> by Group, and another to make <scene name="/12/3456/Sample/2">a transparent representation</scene> of the protein. You can make your own scenes on SAT starting from scratch or loading and editing one of these sample scenes. | ||
</StructureSection> | </StructureSection> | ||
| - | == References for further information on the pheromone binding protein from Apis mellifera == | + | == Contributors == |
| + | |||
| + | |||
| + | |||
| + | Sophie Morin & Mathias Buytaert | ||
| + | |||
| + | == References for further information on the pheromone binding protein from ''Apis mellifera'' == | ||
<references/> | <references/> | ||
Current revision
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
Antennal Specific Protein-1 from Apis mellifera (AmelASP1) with a serendipitous ligand at pH 5.5
| |||||||||||
Contributors
Sophie Morin & Mathias Buytaert
References for further information on the pheromone binding protein from Apis mellifera
- ↑ http://www.genome.jp/dbget-bin/www_bget?pdb:3FE6
- ↑ Pesenti ME, Spinelli S, Bezirard V, Briand L, Pernollet JC, Tegoni M, Cambillau C. Structural basis of the honey bee PBP pheromone and pH-induced conformational change. J Mol Biol. 2008 Jun 27;380(1):158-69. Epub 2008 Apr 27. PMID:18508083 doi:10.1016/j.jmb.2008.04.048
- ↑ Han L, Zhang YJ, Zhang L, Cui X, Yu J, Zhang Z, Liu MS. Operating mechanism and molecular dynamics of pheromone-binding protein ASP1 as influenced by pH. PLoS One. 2014 Oct 22;9(10):e110565. doi: 10.1371/journal.pone.0110565., eCollection 2014. PMID:25337796 doi:http://dx.doi.org/10.1371/journal.pone.0110565
- ↑ Lartigue A, Gruez A, Briand L, Blon F, Bezirard V, Walsh M, Pernollet JC, Tegoni M, Cambillau C. Sulfur single-wavelength anomalous diffraction crystal structure of a pheromone-binding protein from the honeybee Apis mellifera L. J Biol Chem. 2004 Feb 6;279(6):4459-64. Epub 2003 Oct 31. PMID:14594955 doi:10.1074/jbc.M311212200
- ↑ Pesenti ME, Spinelli S, Bezirard V, Briand L, Pernollet JC, Campanacci V, Tegoni M, Cambillau C. Queen bee pheromone binding protein pH-induced domain swapping favors pheromone release. J Mol Biol. 2009 Jul 31;390(5):981-90. Epub 2009 May 28. PMID:19481550 doi:10.1016/j.jmb.2009.05.067
- ↑ Han L, Zhang YJ, Zhang L, Cui X, Yu J, Zhang Z, Liu MS. Operating mechanism and molecular dynamics of pheromone-binding protein ASP1 as influenced by pH. PLoS One. 2014 Oct 22;9(10):e110565. doi: 10.1371/journal.pone.0110565., eCollection 2014. PMID:25337796 doi:http://dx.doi.org/10.1371/journal.pone.0110565
- ↑ http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=3fe6&template=ligands.html&l=1.1
- ↑ http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=3fe6&template=ligands.html&l=2.1
- ↑ http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=3fe6&template=ligands.html&o=METAL&l=1.1