Sandbox CYPMetabolism
From Proteopedia
(Difference between revisions)
| (8 intermediate revisions not shown.) | |||
| Line 59: | Line 59: | ||
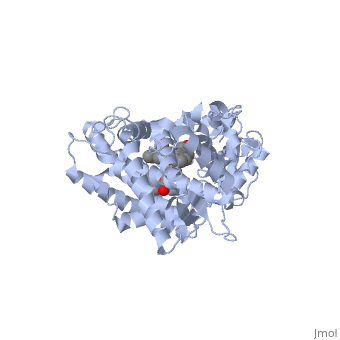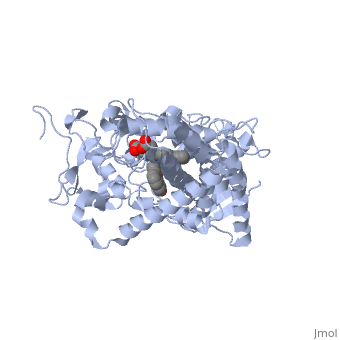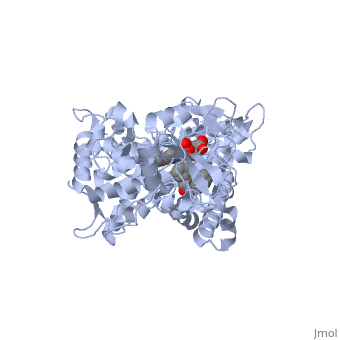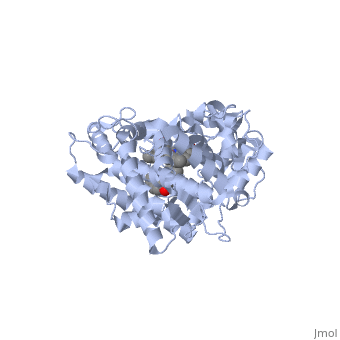
The following scene shows the ligand-free form of <scene name='60/609993/Cyp3a4/1'>CYP3A4</scene>. The next scene shows the <scene name='60/609993/Cyp3a4/15'>binding pocket</scene> of CYP3A4 without a substrate. Examine the size and shape of the cavity by rotating and resizing the molecule. Observe that the cavity extends toward the heme ring and is located more so on the periphery. In order for a drug to be oxidized, it must come quite close to the oxygen atom held by the heme. It is important to realize that a change must occur in order for the binding pocket to be oriented in a way that is conducive to allowing metabolism to occur. | The following scene shows the ligand-free form of <scene name='60/609993/Cyp3a4/1'>CYP3A4</scene>. The next scene shows the <scene name='60/609993/Cyp3a4/15'>binding pocket</scene> of CYP3A4 without a substrate. Examine the size and shape of the cavity by rotating and resizing the molecule. Observe that the cavity extends toward the heme ring and is located more so on the periphery. In order for a drug to be oxidized, it must come quite close to the oxygen atom held by the heme. It is important to realize that a change must occur in order for the binding pocket to be oriented in a way that is conducive to allowing metabolism to occur. | ||
| - | Now, take a look at this scene showing <scene name='60/609993/Cyp3a4/18'>CYP3A4 bound to erythromycin</scene> (PDB entry [[2j0d]]) | + | Now, take a look at this scene showing <scene name='60/609993/Cyp3a4/18'>CYP3A4 bound to erythromycin</scene> (PDB entry [[2j0d]]). The cavity is now in closer proximity to the heme ring and the shape of the cavity has changed as well. In this case, CYP3A4 has undergone a conformational change as a result of binding to erythromycin. In comparing the bound and unbound structures, it looks as though the enzyme has adopted a conformation that allows the drug to occupy the pocket more specifically tailored to its size and shape for a tighter fit. You will also notice that there is an opening in the cavity where erythromycin entered. This, again, is induced fit. |
| - | + | ||
| Line 68: | Line 67: | ||
It has been demonstrated that two or more smaller molecules may bind within an active site at the same time. In this case, drug metabolism can, strangely enough, actually be increased. It has been proposed that when only one molecule of a smaller drug (let's call this Drug A) is bound to the active site, that the extent of Drug A 's metabolism can be minimal due to the relatively large cavity. One explanation may be that Drug A isn't held in a sufficient orientation to the heme Iron. For optimum metabolism, the heme should bond with an Oxygen of the ligand. However, when two molecules of a smaller drug bind at the same time, one molecule may help in forcing the other molecule to retain proper orientation; thus, improving catalytic efficiency. This theory of two drugs binding simultaneously to a CYP may influence its pharmacokinetics is illustrated by the following structure of ketoconazole bound to a CYP enzyme ([[2v0m]]) <ref>PMID:16954191</ref>. | It has been demonstrated that two or more smaller molecules may bind within an active site at the same time. In this case, drug metabolism can, strangely enough, actually be increased. It has been proposed that when only one molecule of a smaller drug (let's call this Drug A) is bound to the active site, that the extent of Drug A 's metabolism can be minimal due to the relatively large cavity. One explanation may be that Drug A isn't held in a sufficient orientation to the heme Iron. For optimum metabolism, the heme should bond with an Oxygen of the ligand. However, when two molecules of a smaller drug bind at the same time, one molecule may help in forcing the other molecule to retain proper orientation; thus, improving catalytic efficiency. This theory of two drugs binding simultaneously to a CYP may influence its pharmacokinetics is illustrated by the following structure of ketoconazole bound to a CYP enzyme ([[2v0m]]) <ref>PMID:16954191</ref>. | ||
| - | Ketoconazole is an anti-fungal drug that can have unusual pharmacokinetics; its apparent plasma concentration does not reflect what we would traditionally expect when considering the dose given. In the structure shown next, <scene name='60/609993/Cyp3a4/6'> two molecules of ketoconazole</scene> are bound to the CYP. You | + | Ketoconazole is an anti-fungal drug that can have unusual pharmacokinetics; its apparent plasma concentration does not reflect what we would traditionally expect when considering the dose given. In the structure shown next, <scene name='60/609993/Cyp3a4/6'> two molecules of ketoconazole</scene> are bound to the CYP. You will also notice that both molecules play a role in the drug being metabolized when the <scene name='60/609993/Cyp3a4/11'>binding pocket</scene> is displayed. One of the ketoconazole molecules is bound directly to the heme ring, while the second molecule has taken up residence in the pocket and appears to be ensuring the first one remain in place. You can see the relationship of the two ketoconazole molecules better if we <scene name='60/609993/Cyp3a4/10'>remove the protein</scene>. The unusual pharmacokinetics of ketoconazole may be explained by the fact that as its plasma concentration increases, the activity of the enzyme is altered due to two drugs now being bound. |
== Irreversible inhibition of CYP450s== | == Irreversible inhibition of CYP450s== | ||
| - | When we examined CYP1A2 above, the flavone inhibited the enzyme simply by virtue of having such complementary features to the binding site. It binds so tightly that it physically prevents other drugs from binding to it. Another way that a drug can inhibit CYP450 enzymes is by formation of a covalent bond that deactivates the active site. This happens with a well-known inhibitor of CYP3A4, <scene name='60/609993/Ritonavir/1'>ritonavir</scene>. Ritonavir is a HIV protease inhibitor routinely prescribed in combination with other antivirals. Its efficacy as part of a drug "cocktail" stems from the fact that it is a potent "irreversible inhibitor" of CYP3A4. Irreversible inhibition differs from most cases of competitive inhibition in that the enzyme is permanently deactivated, and must be re-synthesized by the cell. | + | When we examined CYP1A2 above, the flavone inhibited the enzyme simply by virtue of having such complementary features to the binding site. It binds so tightly that it physically prevents other drugs from binding to it. Another way that a drug can inhibit CYP450 enzymes is by formation of a covalent bond that deactivates the active site. This happens with a well-known inhibitor of CYP3A4, <scene name='60/609993/Ritonavir/1'>ritonavir</scene>. Ritonavir is a HIV protease inhibitor routinely prescribed in combination with other antivirals. Its efficacy as part of a drug "cocktail" stems from the fact that it is a potent "irreversible inhibitor" of CYP3A4. Irreversible inhibition differs from most cases of competitive inhibition in that the enzyme is permanently deactivated, and must be re-synthesized by the cell. In contrast to erythromycin, there has been a covalent bond formed between the <scene name='60/609993/Ritonavir/3'>thiazole ring</scene> of the drug and the heme ring. Do you see how the thiazole has bound to the heme? |
In this case, we have taken advantage of the inhibition of CYP3A4 to prevent it from metabolizing the other antivirals of which ritonavir is co-administered. As you may expect though, extreme caution must be taken to prevent toxicity when other medications are taken concurrently. This is relatively easy to control when only one pharmacy is dispensing all of the medications a patient is prescribed. When more than one pharmacy is involved, however, serious interactions may be overlooked due to one pharmacy being unaware of the medications a patient is receiving from the other pharmacy. | In this case, we have taken advantage of the inhibition of CYP3A4 to prevent it from metabolizing the other antivirals of which ritonavir is co-administered. As you may expect though, extreme caution must be taken to prevent toxicity when other medications are taken concurrently. This is relatively easy to control when only one pharmacy is dispensing all of the medications a patient is prescribed. When more than one pharmacy is involved, however, serious interactions may be overlooked due to one pharmacy being unaware of the medications a patient is receiving from the other pharmacy. | ||
| - | + | In the next section below, we will examine another factor that causes one drug to be metabolized by one CYP, while another might be metabolized by a second one. | |
| - | + | == Active site Volume Affects Drug Selectivity== | |
| + | Some of the factors that determine which particular CYP450 isoform metabolizes a given drug are the shape and size of its active site. As we saw above, induced fit can cause the shape of a binding pocket to change. However, induced fit may not be enough to allow a CYP with a small binding pocket to open up enough to allow larger drugs to bind. The next scene shows | ||
| + | <scene name='60/609993/Cyp1e2/1'>CYP1E2</scene> bound to the enzyme inhibitor 4-methylpyrazole (PDB code [[3e4e]]). If you will now refer back to | ||
| + | <scene name='60/609993/Cyp3a4/18'>erythromycin bound to CYP3A4</scene>, you will notice that, in comparison to CYP1E2, there is quite a dramatic difference in the size and shape of the active site. Keep in mind that although the size and shape of each cavity can change to accommodate different drugs, CYP2E1 cannot expand to the same degree that CYP3A4 can. In fact, CYP2E1 is known to only metabolize drugs that are quite small. A few examples would be ethanol, halothane, and aniline. | ||
Current revision
| |||||||||||