Erythropoietin
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
==Erythropoietin Structure, Function, and History== | ==Erythropoietin Structure, Function, and History== | ||
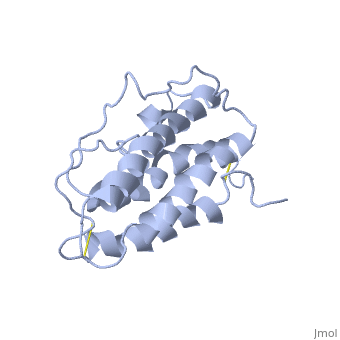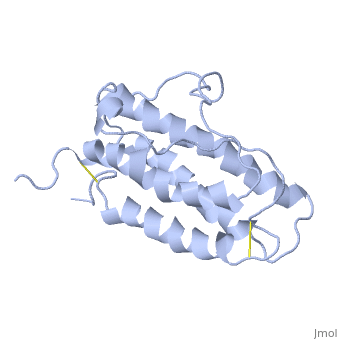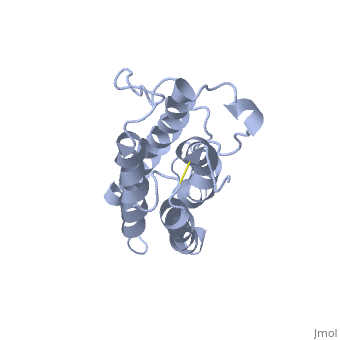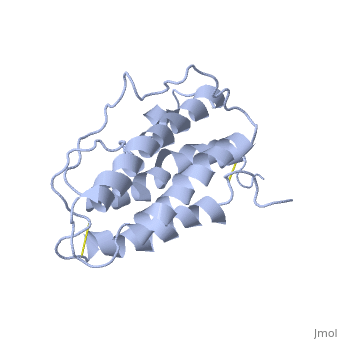
| - | <StructureSection load='1buy' size=' | + | <StructureSection load='1buy' size='350' side='right' caption='Human erythropoietin NMR structure (PDB code [[1buy]]).' scene=> |
==Introduction== | ==Introduction== | ||
| Line 7: | Line 7: | ||
---- | ---- | ||
| - | '''Erythropoietin''' (EPO) is a hormone produced in the kidneys that stimulates the formation of red blood cells. EPO is a glycoprotein that is stimulated when the levels of O2 are abnormally low. This event signals more red blood cells to made from the erythrocytes. Abnormal levels of erythropoietin can be associated with bone marrow disorders, kidney disease, or a synthesized recombinant form that has been injected into the blood stream. Synthesized recombinant EPO has made many headlines in the past few years, due to its use to by Tour de France athletes. They used EPO to illegally dope their blood and increase the amount of oxygen that can be consumed by the body at the time of administration thus increasing endurance. They used EPO because there was no test at the time that could differentiate between naturally produced EPO and the form that was injected. | + | '''Erythropoietin''' (EPO) is a [[Hormones|hormone]] produced in the kidneys that stimulates the formation of red blood cells. EPO is a glycoprotein that is stimulated when the levels of O2 are abnormally low. This event signals more red blood cells to made from the erythrocytes. Abnormal levels of erythropoietin can be associated with bone marrow disorders, kidney disease, or a synthesized recombinant form that has been injected into the blood stream. Synthesized recombinant EPO has made many headlines in the past few years, due to its use to by Tour de France athletes. They used EPO to illegally dope their blood and increase the amount of oxygen that can be consumed by the body at the time of administration thus increasing endurance. They used EPO because there was no test at the time that could differentiate between naturally produced EPO and the form that was injected. |
==History== | ==History== | ||
| Line 34: | Line 34: | ||
and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.</ref>. The intracellular domain of this receptor does not possess any enzymatic activity like other receptors. When EPO comes in contact with the extracellular domains form a ligand bond. The extracellular sinding site 1 and Binding site 2 are composed of <scene name='58/583377/Eporeceptord1d2/1'>D1 and D2</scene> <ref>Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.</ref>. When EPO binds, all loops on D1 and D2 of binding site one form a bind with EPO. However loop 4 of D1 on binding site 2 does not participate in the binding of EPO <ref>PMID: 9774108</ref>. After the biniding of EPO, 8 tyrosine residues are phosphoralated which activates the <scene name='58/583377/Jak2/2'>Jak2 kinase</scene> <ref>Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology | and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.</ref>. The intracellular domain of this receptor does not possess any enzymatic activity like other receptors. When EPO comes in contact with the extracellular domains form a ligand bond. The extracellular sinding site 1 and Binding site 2 are composed of <scene name='58/583377/Eporeceptord1d2/1'>D1 and D2</scene> <ref>Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.</ref>. When EPO binds, all loops on D1 and D2 of binding site one form a bind with EPO. However loop 4 of D1 on binding site 2 does not participate in the binding of EPO <ref>PMID: 9774108</ref>. After the biniding of EPO, 8 tyrosine residues are phosphoralated which activates the <scene name='58/583377/Jak2/2'>Jak2 kinase</scene> <ref>Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology | ||
and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.</ref>. This kinase helps regulate the transcription of different genes and expression of other proteins. | and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.</ref>. This kinase helps regulate the transcription of different genes and expression of other proteins. | ||
| + | |||
| + | See also [[Erythropoietin receptor]] | ||
| + | </StructureSection> | ||
==3D structures of erythropoietin== | ==3D structures of erythropoietin== | ||
Current revision
Erythropoietin Structure, Function, and History
| |||||||||||
3D structures of erythropoietin
Updated on 11-December-2019
1cn4 – hEP (mutant) + EP receptor – human
1buy – hEP (mutant) - NMR
1eer – hEP (mutant) + EP receptor (mutant)
References
- ↑ Kawakita M, Ogawa M, Goldwasser E, Miyake T. Characterization of human megakaryocyte colony-stimulating factor in the urinary extracts from patients with aplastic anemia and idiopathic thrombocytopenic purpura. Blood. 1983 Mar;61(3):556-60. PMID:6600633
- ↑ Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007 Mar;78(3):183-205. Epub 2007 Jan 23. PMID:17253966 doi:http://dx.doi.org/10.1111/j.1600-0609.2007.00818.x
- ↑ Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007 Mar;78(3):183-205. Epub 2007 Jan 23. PMID:17253966 doi:http://dx.doi.org/10.1111/j.1600-0609.2007.00818.x
- ↑ Jelkmann W. Erythropoietin: structure, control of production, and function. Physiol Rev. 1992 Apr;72(2):449-89. PMID:1557429
- ↑ Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007 Mar;78(3):183-205. Epub 2007 Jan 23. PMID:17253966 doi:http://dx.doi.org/10.1111/j.1600-0609.2007.00818.x
- ↑ Erslev, A. J., and J. Caro. "Physiologic and molecular biology of erythropoietin." Medical oncology and tumor pharmacotherapy 3.3-4 (1986): 159-164.
- ↑ Erslev, A. J., and J. Caro. "Physiologic and molecular biology of erythropoietin." Medical oncology and tumor pharmacotherapy 3.3-4 (1986): 159-164.
- ↑ Erslev, A. J., and J. Caro. "Physiologic and molecular biology of erythropoietin." Medical oncology and tumor pharmacotherapy 3.3-4 (1986): 159-164.
- ↑ Jelkmann W. Erythropoietin after a century of research: younger than ever. Eur J Haematol. 2007 Mar;78(3):183-205. Epub 2007 Jan 23. PMID:17253966 doi:http://dx.doi.org/10.1111/j.1600-0609.2007.00818.x
- ↑ Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.
- ↑ Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.
- ↑ Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.
- ↑ Syed RS, Reid SW, Li C, Cheetham JC, Aoki KH, Liu B, Zhan H, Osslund TD, Chirino AJ, Zhang J, Finer-Moore J, Elliott S, Sitney K, Katz BA, Matthews DJ, Wendoloski JJ, Egrie J, Stroud RM. Efficiency of signalling through cytokine receptors depends critically on receptor orientation. Nature. 1998 Oct 1;395(6701):511-6. PMID:9774108 doi:http://dx.doi.org/10.1038/26773
- ↑ Constantinescu, Stefan N., Saghi Ghaffar, Harvey F. Lodish. The Erythropoietin Receptor: Structure, Activation and Intracellular Signal Transduction. Trends in Endocrinology and Metabolism, Volume10, Issue 1, 1January 1999, Pages 18-23.