RA Mediated T-reg Differentiation
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
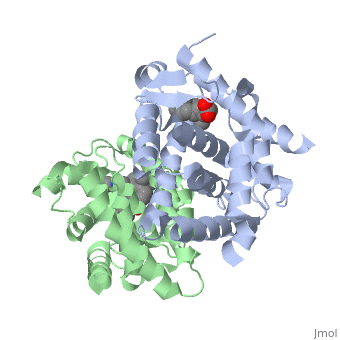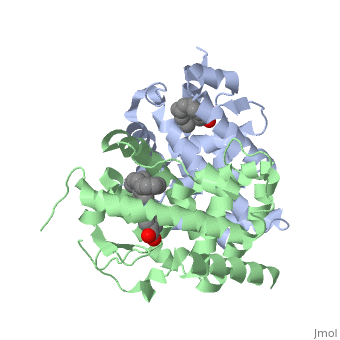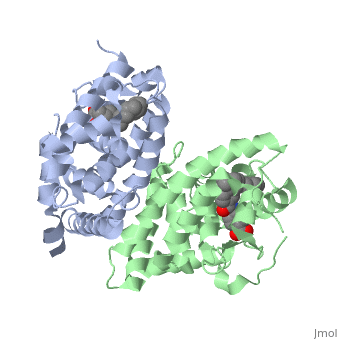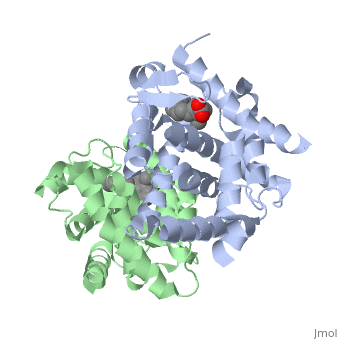
| - | <StructureSection load='1dkf' size='400' side='right' scene='' caption='Mouse retinoid X receptor-α ligand-binding domain (grey) complex with retinoic acid receptor-α ligand-binding domain (green) (PDB code [[1dkf]]). '> | + | <StructureSection load='1dkf' size='400' side='right' scene='' caption='Mouse retinoid X receptor-α ligand-binding domain (grey) complex with retinoic acid receptor-α ligand-binding domain (green), oleic acid and benzoic acid derivative (PDB code [[1dkf]]). '> |
==Introduction== | ==Introduction== | ||
| - | T-regulatory cells (T-regs) are a small subset of CD4+ T-cells that exhibit strong down regulation of immune system activity in their local environment. They are distinguished from other CD4+ T-cells by the expression of FOXP3, a gene regulator. <ref> PMID: 19410687 </ref> The exact mechanisms used by T-regs to down regulate the immune system has not yet been clearly elucidated. These cells have been shown to differentiate from CD4+ T-helper cells upon activation and exposure to the following cytokines: tumor growth factor β (TGF-β), Interleukin-2 (IL-2) and retinoic acid (RA). <ref> PMID: 21839265 </ref> Both TGF-β and IL-2 are used in other immune system differentiation, however, RA has been shown to bias T-cells to the T-reg phenotype. <ref> PMID: 21839265 </ref> When acting upon T-reg cells, RA acts as the ligand for the Retinoic Acid Receptor-α (RARα) / Retinoid X Receptor-α (RXRα) heterodimer. This heterodimer is of the nuclear receptor family, and each chain consists of the same three part structure: a Ligand binding domain (LBD), a DNA binding domain (DBD), and a hinge region connecting the two binding domains. <ref> PMID: 10406480 </ref> | + | T-regulatory cells (T-regs) are a small subset of CD4+ T-cells that exhibit strong down regulation of immune system activity in their local environment. They are distinguished from other CD4+ T-cells by the expression of FOXP3, a gene regulator. <ref> PMID: 19410687 </ref> The exact mechanisms used by T-regs to down regulate the immune system has not yet been clearly elucidated. These cells have been shown to differentiate from CD4+ T-helper cells upon activation and exposure to the following cytokines: tumor growth factor β (TGF-β), Interleukin-2 (IL-2) and retinoic acid (RA). <ref> PMID: 21839265 </ref> Both TGF-β and IL-2 are used in other immune system differentiation, however, RA has been shown to bias T-cells to the T-reg phenotype. <ref> PMID: 21839265 </ref> When acting upon T-reg cells, RA acts as the ligand for the Retinoic Acid Receptor-α (RARα) / Retinoid X Receptor-α (RXRα) heterodimer. This heterodimer is of the [[Nuclear receptors|nuclear receptor family]], and each chain consists of the same three part structure: a Ligand binding domain (LBD), a DNA binding domain (DBD), and a hinge region connecting the two binding domains. <ref> PMID: 10406480 </ref> |
| + | |||
| + | See also [[Intracellular receptors]] | ||
==Ligand Binding Domain== | ==Ligand Binding Domain== | ||
| Line 50: | Line 52: | ||
==Biological Significance== | ==Biological Significance== | ||
Since T-regulatory cells are so highly regulated in the body, elucidating the exact mechanism of activation can show how these immune processes work, and use them in the treatment of disease. Two clear mechanisms of regulation arise from the studies, both which are related to the heterodimer itself. First, the ligand specificity for RA in both molecules allows for specific signaling of these molecules. RA is not normally expressed in cells, and therefore will limit when this heterodimer is activated. Likewise, the propensity for the heterodimer to associate with DR-5 repeats limits the number of genes it will activate to a select few. All of this in addition to the other cytokines necessary, TGF-β and IL-2, show the complex mechanisms regulating the differentiation of T-helper cells into T-regulatory cells. | Since T-regulatory cells are so highly regulated in the body, elucidating the exact mechanism of activation can show how these immune processes work, and use them in the treatment of disease. Two clear mechanisms of regulation arise from the studies, both which are related to the heterodimer itself. First, the ligand specificity for RA in both molecules allows for specific signaling of these molecules. RA is not normally expressed in cells, and therefore will limit when this heterodimer is activated. Likewise, the propensity for the heterodimer to associate with DR-5 repeats limits the number of genes it will activate to a select few. All of this in addition to the other cytokines necessary, TGF-β and IL-2, show the complex mechanisms regulating the differentiation of T-helper cells into T-regulatory cells. | ||
| + | |||
| + | ==3D structures of RXR and RAR== | ||
| + | [[Retinoid X receptor]]<br /> | ||
| + | [[Retinoic acid receptor]] | ||
</StructureSection> | </StructureSection> | ||
==References== | ==References== | ||
<references /> | <references /> | ||
Current revision
| |||||||||||
References
- ↑ Ochs HD, Oukka M, Torgerson TR. TH17 cells and regulatory T cells in primary immunodeficiency diseases. J Allergy Clin Immunol. 2009 May;123(5):977-83; quiz 984-5. PMID:19410687 doi:10.1016/j.jaci.2009.03.030
- ↑ Moore C, Fuentes C, Sauma D, Morales J, Bono MR, Rosemblatt M, Fierro JA. Retinoic acid generates regulatory T cells in experimental transplantation. Transplant Proc. 2011 Jul-Aug;43(6):2334-7. PMID:21839265 doi:10.1016/j.transproceed.2011.06.057
- ↑ Moore C, Fuentes C, Sauma D, Morales J, Bono MR, Rosemblatt M, Fierro JA. Retinoic acid generates regulatory T cells in experimental transplantation. Transplant Proc. 2011 Jul-Aug;43(6):2334-7. PMID:21839265 doi:10.1016/j.transproceed.2011.06.057
- ↑ Kumar R, Thompson EB. The structure of the nuclear hormone receptors. Steroids. 1999 May;64(5):310-9. PMID:10406480
- ↑ Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000 Feb;5(2):289-98. PMID:10882070
- ↑ Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000 Feb;5(2):289-98. PMID:10882070
- ↑ Bourguet W, Vivat V, Wurtz JM, Chambon P, Gronemeyer H, Moras D. Crystal structure of a heterodimeric complex of RAR and RXR ligand-binding domains. Mol Cell. 2000 Feb;5(2):289-98. PMID:10882070
- ↑ Zhao Q, Chasse SA, Devarakonda S, Sierk ML, Ahvazi B, Rastinejad F. Structural basis of RXR-DNA interactions. J Mol Biol. 2000 Feb 18;296(2):509-20. PMID:10669605 doi:10.1006/jmbi.1999.3457
- ↑ Zhao Q, Chasse SA, Devarakonda S, Sierk ML, Ahvazi B, Rastinejad F. Structural basis of RXR-DNA interactions. J Mol Biol. 2000 Feb 18;296(2):509-20. PMID:10669605 doi:10.1006/jmbi.1999.3457
- ↑ Umesono K, Murakami KK, Thompson CC, Evans RM. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255-66. PMID:1648450
Proteopedia Page Contributors and Editors (what is this?)
William Bailey, Alexander Berchansky, Michal Harel, Jaime Prilusky