We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 970
From Proteopedia
(Difference between revisions)
| (92 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | < | + | == '''Calcium ATPase''' == |
| + | |||
| + | <StructureSection load='1SU4' size='350' side='right' caption='3D Structure of the SERCA pump resolved with x-ray cristallography' scene='Insert optional scene name here'> | ||
== Introduction == | == Introduction == | ||
| - | All living organisms depend on P-type ATPase to pump | + | |
| - | + | All living organisms depend on P-type ATPase to pump cations across membranes. P-type ATPases are distinct from other ATPases because there is an autophosphorylation step in their catalytic cycle. They play a fundamental role in organisms metabolism and physiology. Ca2+ ATPase is one type of P-type ATPases which transports calcium ions across membranes against a concentration gradient. These pumps clear the cytoplasm of the calcium, which is a second messenger. It's very important to keep a low concentration of calcium in the cell for a good cell signaling. The hydrolysis of ATP is necessary for the pump's functioning. For each ATP hydrolyzed, it transfers two calcium ions through the membrane, and two or three hydrogen ions in the opposite direction. | |
| - | The hydrolysis of | + | |
== Structural Highlights == | == Structural Highlights == | ||
| - | The calcium ATPase is a protein composed of | + | The calcium ATPase is a protein composed of 994 aminoacids. |
| - | The | + | The <scene name='60/604489/Secondary_structure/1'>secondary structure</scene> of the protein is composed of helical regions (47%), beta sheet regions (16%) and loops. There are <scene name='60/604489/10_transmembrane_helices/1'>10 transmembrane alpha helices</scene>, and three of them line a channel that spans the lipid bilayer and that allows calcium to pass through membranes. It also contains too cytoplasmic loops between the transmembrane helices. When the protein is not phosphorylated, two of the transmembrane helices are disrupted and form a cavity that can bind two molecules of calcium. |
| - | The '''transmembrane | + | The protein is divided in <scene name='60/604489/The_4_domains_of_the_pump/1'>4 regions</scene>. The <scene name='60/604489/Transmembrane_domain/1'>transmembrane region</scene> of the protein contains the channel that spans the lipid bilayer, and the calcium binding cavity. |
| - | The two cytoplasmic loops form three separate domains. The '' | + | The two cytoplasmic loops form three separate domains. The <scene name='60/604489/Nucleotide_binding_domain/1'>nucleotide binding domain (N)</scene> contains the site where ATP binds to the protein. The <scene name='60/604489/P_domain/1'>phosphorylation domain (P)</scene> contains an Aspartate residue (<scene name='60/604489/Asp_351/1'>Asp 351</scene>) that can be phosphorylated. Finally, the <scene name='60/604489/Actuator_domain/1'>actuator domain (A)</scene> is involved in the transmission of major conformational changes. The phosphorylation and the nucleotide binding domains form the <scene name='60/604489/Catalytic_site/1'>catalytic site</scene> of the protein<ref name="first">Benjamin Lewin, 2007 - Cells - Jones & Bartlett Learning</ref>. |
| - | == | + | == Mechanism of action == |
| - | The architecture of calcium ATPase (determined by X-Ray crystallography) | + | |
| + | The architecture of calcium ATPase (determined by X-Ray crystallography) allows to understand mechanisms by which the energy of ATP is coupled to the calcium transport across a membrane. | ||
| + | |||
| + | The first step of the calcium pump catalytic cycle is the cooperative binding of <scene name='60/604489/Calcium_molecules/1'>two calcium ions</scene> in the calcium binding cavity. Then, ATP binds to the ATP binding site (nucleotide binding domain) and transfers its γ-phosphate to the aspartic acid 351 (phosphorylation domain). That creates a acid-stable aspartyl phosphate intermediate. The phosphorylation of Asp351 allows a large conformational changes in cytoplasmic domains: the nucleotide binding domain and the phosphorylation domain are brought into close proximity. This rearrangement causes a 90° rotation of the actuator domain, which leads to a rearrangement of the transmembrane helices. This rearrangement alters the affinity of the protein for the calcium and disrupts the calcium binding cavity. Calcium is released in the lumen of the endoplasmic reticulum/Golgi Apparatus or outside the cell. After releasing calcium, two or three protons bind to the transport sites (charges compensation) and the aspartyl phosphate is hydrolyzed to complete the cycle. <ref name = "four">Marisa Brini , Ernesto Carafoli, 2009 - ''Calcium pumps in health and disease'' - Physiological Reviews</ref> | ||
| + | |||
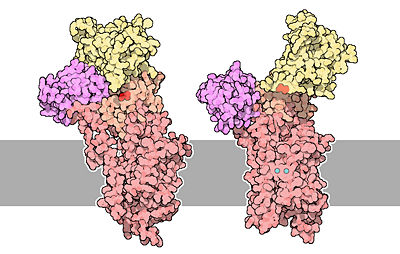
| + | [[Image:51-TheCalciumPumps-calcium-pumps.jpg|400px|center|]] | ||
| + | The structure on the left is the empty state (free from calcium). Two calcium ions are shown in blue and Asp351 is shown in red.<ref name="second"> David Goodsell, 2004 - Calcium pump molecul of the month - PDB, doi: 10.2210/rcsb_pdb/mom_2004_3</ref> | ||
| + | |||
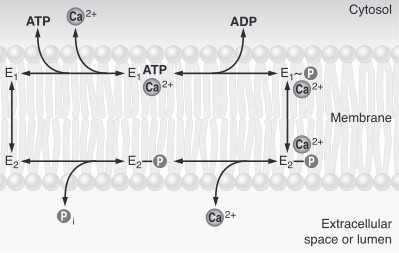
| + | To sum up, calcium pumps have two conformations, E1 and E2. These two conformations are characterized by different specificity for ion binding. When the pump is in the E1 state, it has high calcium affinity and interacts with calcium at one side of the membrane. In the E2 state, the enzyme has a lower calcium affinity and that leads to the release of the ion at the opposite side. E1 has the calcium binding site oriented toward the cytoplasm. E2 has the calcium binding site oriented toward the lumen of the endoplasmic reticulum or toward the extracellular space<ref name="third">Thomas D.Pollard and William C. Earnshaw, - ''Membrane, structure and function'' - Cell Biology (second edition), p.133-136</ref>. The phosphorylated intermediate, E1 can phosphorylate ADP, whereas E2 can only react with water<ref>David H.MacLennan, William J.Rice and N. Michael Green, 1997 - ''The Mechanism of Ca2+ Transport by Sarco(Endo)plasmic Reticulum Ca2+-ATPases'' - The Journal of Biological Chemistry, p.272, 28815-28818, http://www.jbc.org/content/272/46/28815.full.html</ref>. In the absence of calcium, calcium pumps are mainly in E1 state. | ||
| + | |||
| + | |||
| + | [[Image:Etat.jpg|center|]]<ref name = "four">Marisa Brini , Ernesto Carafoli, 2009 - ''Calcium pumps in health and disease'' - Physiological Reviews</ref> | ||
| - | '''The first step of the calcium pump catalytic cycle is the cooperative binding of two calcium ions in the calcium binding cavity. Then, ATP binds to the ATP binding site (nucleotide binding domain) and transfers its γ-phosphate to the aspartic acide 351 (phosphorylation domain). That creates a acid-stable aspartyl phosphate intermediate. The phosphorylation of Asp351 allows a large conformational changes in cytoplasmic domains: the nucleotide binding domain and the phosphorylation domain are brought into close proximity. This rearrangement causes a 90° rotation of the activator domain, which leads to a rearrangement of the transmembrane helices. This rearrangement alters the affinity of the protein for the calcium and disrupts the calcium binding cavity. Calcium is released in the lumen of the endoplasmic reticulum/Golgi Apparatus or outside the cell. After releasing calcium, two protons are bound to the transport sites (charges compensation) and the aspartyl phosphate is hydrolyzed to complete the cycle.''' | ||
| - | In the cytoplasm, the ATP binds to the cytosolic loop that connects transmembrane domains four and five (ATP binding site). It transfers its γ-phosphate to the aspartic acide 351 (phosphorylation site) and creates a acid-stable aspartyl phosphate intermediate. The binding of ATP is initiated by the cooperative fixing of two calcium ions to the transport site. The phosphorylation of Asp351 allows a large conformational changes in cytoplasmic domains which closes the ion gates from the cytoplasm and alters the affinity of the protein for the calcium. After releasing calcium (in the lumen of cytoplasm or out side the cell), two protons are bound to the transport sites (charges compensation) and the aspartyl phosphate is hydrolyzed to complete the cycle. | ||
| - | To sum up, calcium pumps have two conformations, E1 and E2. These two conformations are characterized by different specificity for ion binding. When the pump is in the E1 state, it has high calcium affinity and interacts with calcium at one side of the membrane. In the E2 state, the enzyme has a lower calcium affinity and that leads to the release of the ion at the opposite side. E1 has the calcium binding site oriented toward the cytoplasm. E2 has the calcium binding site oriented toward the lumen of the endoplasmic reticulum or toward the extracellular background. | ||
== Biological Function and Localisation == | == Biological Function and Localisation == | ||
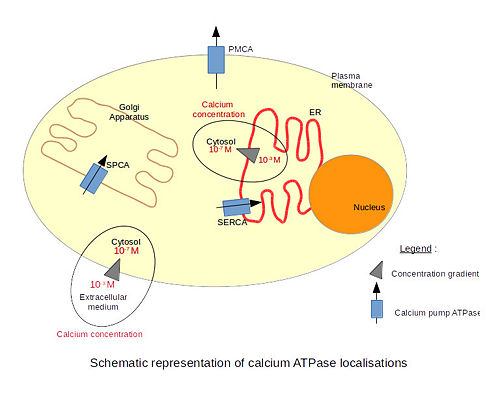
| + | There are 3 types of calcium ATPases depending on their localization. The '''SERCA''' (Sarco/Endoplasmic Reticulum Calcium ATPase) is localized in the endoplasmic reticulum membranes, including the nuclear envelope. Only the structure of the SERCA has been resolved by x-ray crystallography. The '''PMCA''' (Plasma Membrane Calcium ATPase) is localized in plasma membranes. The '''SPCA''' (Secretory Pathway Calcium ATPase) is localized in the Golgi apparatus membranes. The particularity of this pump is that it is also able to transport Mn2+ ions. | ||
| - | + | [[Image:ATPases.jpg|500px|center|]] | |
| - | + | ||
| - | + | ||
| - | + | Calcium is a very important molecule for cell signalling. Eucaryotic cells need to maintain a very low calcium concentration in their cytosol. The extracellular calcium concentration is much higher. That difference of concentrations across the membrane creates a gradient of concentration and allows the signalling to be very effective. Indeed, even a very small influx of calcium significantly increases the concentration of calcium inside the cell. Therefore, calcium pumps are very important to maintain the calcium concentration gradient and to remove calcium from the cell after signalling. | |
| - | + | Calcium is involved in many physiological processes such as programmed cell death, fertilization, gene transcription, secretion (including neurotransmitter secretion) etc. For example, the SERCA pump is mainly found in skeletal muscle cells and cardiac cells. It is involved in the relaxation of skeletal muscle cells. Those cells contain a special endoplasmic reticulum called the sarcoplasmic reticulum which is the place of calcium storage. After contraction, calcium ions are transported from the cytoplasm into the sarcoplasmic reticulum through the SERCA pump. This causes the relaxation of the muscle cells because the cytosolic concentration of calcium decreases. The SERCA pump works together with the PMCA pump to export calcium ions from the cytosol and to set the resting level of the cytosolic calcium concentration<ref name="first">Benjamin Lewin, 2007 - Cells - Jones & Bartlett Learning</ref>. | |
| - | + | ||
| - | Marianela G.Dalghi, Marisa M.Fernández, Mariela Ferreira-Gomes, Irene C.Mangialavori, Emilio L.Malchiodi, Emanuel E.Strehler and Juan Pablo F.C.Rossi, 2013 - ''Plasma Membrane Calcium ATPase Activity Is Regulated by Actin Oligomers through Direct Interaction'' - The Journal of Biological Chemistry, p.288, 23380-23393 | ||
| + | == Regulations == | ||
| + | There are different kinds of calcium ATPase regulations. For example, the <scene name='60/604489/Phospholamban/2'>phospholamban</scene> (PLN or PLB) and the <scene name='60/604489/Sarcolipin/1'>sarcolipin</scene> are membrane proteins that regulate the calcium pump in the cardiac muscle and in skeletal muscle cells. These two proteins are close homologous and play the same role. The phospholamban is a phosphoprotein that can be phosphorylated at three distinct sites by various protein kinases (PKA, PKC, CamK...). The phosphorylation state is mediated through beta-adrenergic stimulation. In unphosphorylated state, the phospholamban inhibits the activity of the calcium pump in cardiac and skeletal muscle cells by decreasing the apparent affinity of the ATPase for calcium. The phosphorylation of the protein results in the dissociation of the protein from the ATPase<ref>Marianela G.Dalghi, Marisa M.Fernández, Mariela Ferreira-Gomes, Irene C.Mangialavori, Emilio L.Malchiodi, Emanuel E.Strehler and Juan Pablo F.C.Rossi, 2013 - ''Plasma Membrane Calcium ATPase Activity Is Regulated by Actin Oligomers through Direct Interaction'' - The Journal of Biological Chemistry, p.288, 23380-23393, http://www.jbc.org/content/288/32/23380.full.</ref>. | ||
| - | Marisa Brini and Ernesto Carafoli, 2010 - ''The | + | Another example of regulation is the plasma membrane calcium pump carboxy-terminal tail that contains the calmodulin binding domain (regulatory domain) which acts as an auto-inhibitory domain. The binding of the calmodulin frees the pump from autoinhibition<ref>Marisa Brini and Ernesto Carafoli, 2010 - ''The plasma membrane Ca2+ ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium'' - Cold Spring Harbor Perspectives in Biology, http://cshperspectives.cshlp.org/content/3/2/a004168.full</ref>. |
| - | + | == Dysfunctions and Diseases == | |
| + | Two human genetic diseases caused by mutations in the SERCA pump have been identified : Brody's disease and Darier's disease.<ref name = "four">Marisa Brini , Ernesto Carafoli, 2009 - ''Calcium pumps in health and disease'' - Physiological Reviews</ref> Brody's disease is caused by 2 mutations in the SERCA1 gene called ATP2A1: <scene name='60/604489/Ile_235/1'>Ile235Asn</scene> and <scene name='60/604489/Glu982/1'>Glu982Lys</scene>. <ref>Nyamkhishig Sambuughin, Elena Zvaritch, Natasha Kraeva, Olga Sizova, Erica Sivak, Kelley Dickson, Margaret Weglinski, John Capacchione, Sheila Muldoon, Sheila Riazi, Susan Hamilton, Barbara Brandom and David H. MacLennan, 2014 - ''Exome analysis identifies Brody myopathy in a family diagnosed with malignant hyperthermia susceptibility'' - Molecular Genetics & Genomic Medicine</ref>. Brody's disease is an autosomal recessive myopathy. The symptoms are muscle stiffness, cramps and progressive impairment of muscle relaxation during exercise. Darier's disease is a rare, dominantly inherited disorder that affects the skin producing a variety of types of lesions. It is caused by mutations in the SERCA2 gene (ATP2A2). Defects in the activity or expression level of the pump can cause other dysfunctions such as heart failure or cancer. | ||
| - | + | </StructureSection> | |
| - | + | == References == | |
| + | <references/> | ||
Current revision
Calcium ATPase
| |||||||||||
References
- ↑ 1.0 1.1 Benjamin Lewin, 2007 - Cells - Jones & Bartlett Learning
- ↑ 2.0 2.1 2.2 Marisa Brini , Ernesto Carafoli, 2009 - Calcium pumps in health and disease - Physiological Reviews
- ↑ David Goodsell, 2004 - Calcium pump molecul of the month - PDB, doi: 10.2210/rcsb_pdb/mom_2004_3
- ↑ Thomas D.Pollard and William C. Earnshaw, - Membrane, structure and function - Cell Biology (second edition), p.133-136
- ↑ David H.MacLennan, William J.Rice and N. Michael Green, 1997 - The Mechanism of Ca2+ Transport by Sarco(Endo)plasmic Reticulum Ca2+-ATPases - The Journal of Biological Chemistry, p.272, 28815-28818, http://www.jbc.org/content/272/46/28815.full.html
- ↑ Marianela G.Dalghi, Marisa M.Fernández, Mariela Ferreira-Gomes, Irene C.Mangialavori, Emilio L.Malchiodi, Emanuel E.Strehler and Juan Pablo F.C.Rossi, 2013 - Plasma Membrane Calcium ATPase Activity Is Regulated by Actin Oligomers through Direct Interaction - The Journal of Biological Chemistry, p.288, 23380-23393, http://www.jbc.org/content/288/32/23380.full.
- ↑ Marisa Brini and Ernesto Carafoli, 2010 - The plasma membrane Ca2+ ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium - Cold Spring Harbor Perspectives in Biology, http://cshperspectives.cshlp.org/content/3/2/a004168.full
- ↑ Nyamkhishig Sambuughin, Elena Zvaritch, Natasha Kraeva, Olga Sizova, Erica Sivak, Kelley Dickson, Margaret Weglinski, John Capacchione, Sheila Muldoon, Sheila Riazi, Susan Hamilton, Barbara Brandom and David H. MacLennan, 2014 - Exome analysis identifies Brody myopathy in a family diagnosed with malignant hyperthermia susceptibility - Molecular Genetics & Genomic Medicine