Sandbox Reserved 975
From Proteopedia
| (6 intermediate revisions not shown.) | |||
| Line 2: | Line 2: | ||
== SUMO-conjugating enzyme UBC9 EC:6.3.2. == | == SUMO-conjugating enzyme UBC9 EC:6.3.2. == | ||
| - | + | <StructureSection load='3uin' size='340' side='right' caption='Structure of UBC9' scene=''> | |
==Context== | ==Context== | ||
| - | <StructureSection load='3uin' size='340' side='right' caption='Structure of UBC9' scene=''> | ||
The SUMO-conjugating enzyme UBC9 is involved in ubiquitination of proteins. | The SUMO-conjugating enzyme UBC9 is involved in ubiquitination of proteins. | ||
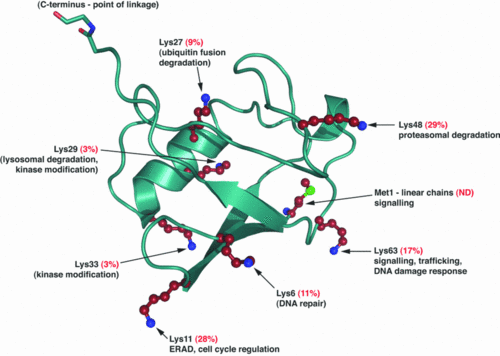
The Ubiquitin is a small protein of 76 aa. | The Ubiquitin is a small protein of 76 aa. | ||
| + | |||
| + | [[Image:Bst0370937a03.gif|500px]] | ||
Ubiquitination is a post translationnal modification where an ubiquitin is attached to a protein. This modification has several consequences, it can lead to the degradation of the protein via the proteasome, it can change the protein localization or it can alter the interaction of the protein with other factors, this is why this modification plays an important role in the control of many cellular processes. | Ubiquitination is a post translationnal modification where an ubiquitin is attached to a protein. This modification has several consequences, it can lead to the degradation of the protein via the proteasome, it can change the protein localization or it can alter the interaction of the protein with other factors, this is why this modification plays an important role in the control of many cellular processes. | ||
| Line 56: | Line 57: | ||
- 5 residues (32-36) form most of a very exposed <scene name='60/604494/Beta_hairpin/1'>β-hairpin</scene> that connects strands β1 and β2. | - 5 residues (32-36) form most of a very exposed <scene name='60/604494/Beta_hairpin/1'>β-hairpin</scene> that connects strands β1 and β2. | ||
| - | - The residues Asp100 and Lys101 form a bulge in a loop (residues 94–102) close to Cys93 | + | - The residues Asp100 and Lys101 form a <scene name='60/604494/Bulge_in_a_loop/1'>bulge in a loop</scene> (residues 94–102) close to Cys93 |
Those insertions provide additionnal binding sites for new substrate without blocking the access to Cys | Those insertions provide additionnal binding sites for new substrate without blocking the access to Cys | ||
| Line 64: | Line 65: | ||
There is a negative patch surrounding the active site that is conserved between all UBCs, it is probably important in the interaction with Ubiquitin and E1, the common substrates of all UBC9s. | There is a negative patch surrounding the active site that is conserved between all UBCs, it is probably important in the interaction with Ubiquitin and E1, the common substrates of all UBC9s. | ||
| - | But there are many particularities in the surface electric potential of UBC9, that probably reflect the specificity for E3 and the protein substrate. The electrostatic dipole is more important for UBC9 than for others UBCs. The positive charged are located on the | + | But there are many particularities in the surface electric potential of UBC9, that probably reflect the specificity for E3 and the protein substrate. The electrostatic dipole is more important for UBC9 than for others UBCs. The positive charged are located on the back face of the molecule. One, for example, is located on the N-terminal region, it is composed of a segment of basic residues separated by nonpolar residues (13RKAWRK18). This <scene name='60/604494/Postive_patch/1'>positive patch is located close to the β-hairpin </scene>, the presence of those two specifities in the same region could be a sign that this site is responsible for the specificity of UBC9 for some particular E3 and protein substrates. |
</StructureSection> | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
| + | - Allan D. Capili and Christopher D. Lima - Structure and analysis of a complex between SUMO and Ubc9 illustrates features of a conserved E2-Ubl interaction. Consulted the 28/12/2014 : http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1940065/ | ||
| + | |||
| + | - Ubiquitin from wikipedia : http://en.wikipedia.org/wiki/Ubiquitin. Consulted the 27/12/2014 | ||
| + | |||
| + | - Harry Tong, Guus Hateboer, Anastassis Perrakis, René Bernards and Titia K. Sixma - Crystal Structure of Murine/Human Ubc9 Provides Insight into the Variability of the Ubiquitin-conjugating System. Consulted the 28/12/2014 : http://www.jbc.org/content/272/34/21381.full | ||
| + | |||
| + | - Uniprot P63279- UBC9_HUMAN - SUMO-conjugating enzyme UBC9 : http://www.uniprot.org/uniprot/P63279. Consulted the 27/12/2014 | ||
Current revision
| This Sandbox is Reserved from 15/11/2014, through 15/05/2015 for use in the course "Biomolecule" taught by Bruno Kieffer at the Strasbourg University. This reservation includes Sandbox Reserved 951 through Sandbox Reserved 975. |
To get started:
More help: Help:Editing |
SUMO-conjugating enzyme UBC9 EC:6.3.2.
| |||||||||||
References
- Allan D. Capili and Christopher D. Lima - Structure and analysis of a complex between SUMO and Ubc9 illustrates features of a conserved E2-Ubl interaction. Consulted the 28/12/2014 : http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1940065/
- Ubiquitin from wikipedia : http://en.wikipedia.org/wiki/Ubiquitin. Consulted the 27/12/2014
- Harry Tong, Guus Hateboer, Anastassis Perrakis, René Bernards and Titia K. Sixma - Crystal Structure of Murine/Human Ubc9 Provides Insight into the Variability of the Ubiquitin-conjugating System. Consulted the 28/12/2014 : http://www.jbc.org/content/272/34/21381.full
- Uniprot P63279- UBC9_HUMAN - SUMO-conjugating enzyme UBC9 : http://www.uniprot.org/uniprot/P63279. Consulted the 27/12/2014