We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Andrea Bauer/Sandbox 956
From Proteopedia
(Difference between revisions)
| (14 intermediate revisions not shown.) | |||
| Line 11: | Line 11: | ||
== Description == | == Description == | ||
| - | Isoprene Synthase is supposed to be a dimeric enzyme which consists of 595 amino acids and has a molecular mass of 68,386 Da. The protein is made up of alpha-helices which form two alpha-helical domains. | + | Isoprene Synthase is supposed to be a dimeric enzyme which consists of 595 amino acids and has a molecular mass of 68,386 Da. The protein is made up of alpha-helices which form two alpha-helical domains.<ref name="Koch">PMID:20624401</ref> |
| - | The <scene name='68/686749/N-terminal_domain/1'>N-terminal domain</scene> of the protein chain is folded similar to class II terpenoid synthases that are made up of (𝛼𝛼)6 barrels | + | The <scene name='68/686749/N-terminal_domain/1'>N-terminal domain</scene> of the protein chain is folded similar to class II terpenoid synthases that are made up of (𝛼𝛼)6 barrels <ref name="Went">PMID:9519404</ref>. |
| - | Up to now there is no catalytic activity known for this domain. Quite the contrary regarding the <scene name='68/686749/C-terminal_domain/1'>C-terminal domain</scene> of the isoprene synthase: This domain shows up an 𝛼-helical class I terpenoid synthase fold and contains the active site which is surrounded by five 𝛼-helices. The active site of the enzyme is located in a deep hydrophobic pocket which ensures a protection of the reaction intermediate from water. | + | Up to now there is no catalytic activity known for this domain. Quite the contrary regarding the <scene name='68/686749/C-terminal_domain/1'>C-terminal domain</scene> of the isoprene synthase: This domain shows up an 𝛼-helical class I terpenoid synthase fold and contains the active site which is surrounded by five 𝛼-helices. The active site of the enzyme is located in a deep hydrophobic pocket which ensures a protection of the reaction intermediate from water.<ref name="Koch"/> |
== Catalyzed Reaction == | == Catalyzed Reaction == | ||
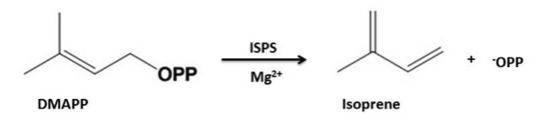
| - | The Isoprene Synthase catalyses the production of isoprene from the substrate dimethylallyl-diphosphate (DMAPP)[http://en.wikipedia.org/wiki/Dimethylallyl_pyrophosphate]. During the reaction inorganic pyrophosphate is eliminated leading to the reaction products isoprene and inorganic pyrophosphate. The release of the pyrophosphate group leads to the generation of an allylic carbocation which is typical of class I terpenoid | + | The Isoprene Synthase catalyses the production of isoprene from the substrate dimethylallyl-diphosphate (DMAPP)[http://en.wikipedia.org/wiki/Dimethylallyl_pyrophosphate]. During the reaction inorganic pyrophosphate is eliminated leading to the reaction products isoprene and inorganic pyrophosphate. The release of the pyrophosphate group leads to the generation of an allylic carbocation which is typical of class I terpenoid syntheses<ref name="Went"/>. The occuring elimination mechanism is syn-periplanar and the leaving diphpsphate group acts as general base. The characteristic DDXXD-sequence motif of class I terpenoid synthases that binds to the diphosphate leaving group via Mg2+-ions facilitates the release of the pyrophosphate group.<ref name="Koch"/> |
| - | [[Image: | + | [[Image:Isoprene_formation.jpg | thumb | upright=3]] |
== Structure == | == Structure == | ||
| - | The hydrophobic active site pocket has a higher affinity towards a 5-carbon substrate rather than to a 10-carbon complex and Van der Waals interactions take place with DMASPP and the isoprenoid <scene name='68/686749/Isoprenoid_moiety/ | + | The hydrophobic active site pocket has a higher affinity towards a 5-carbon substrate rather than to a 10-carbon complex and Van der Waals interactions take place with DMASPP (DMAPP analogous substrate) and the isoprenoid <scene name='68/686749/Isoprenoid_moiety/2'>functional group</scene> of the active site on F338, V341 and F485. |
PcISPS remains in the open conformation while being in the DMASPP complex. | PcISPS remains in the open conformation while being in the DMASPP complex. | ||
It was suggested that the diphosphate leaving group itself serves as general base. So a syn-periplanar elimination reaction was suggested with the development of an intermediate carbocation, which leads to the assumption that the isoprene generation is catalyzed by a substrate-assisted mechanism. | It was suggested that the diphosphate leaving group itself serves as general base. So a syn-periplanar elimination reaction was suggested with the development of an intermediate carbocation, which leads to the assumption that the isoprene generation is catalyzed by a substrate-assisted mechanism. | ||
| - | PcISPS was found to be monomeric in crystallization. However, positive cooperativity has been observed which is unusual for a monomeric enzyme . There was evidence that this cooperativity results from a dimeric quarternary structure, where C-terminal catalytic domains interact to form an isologous dimer. | + | PcISPS was found to be monomeric in crystallization. However, positive cooperativity has been observed which is unusual for a monomeric enzyme . There was evidence that this cooperativity results from a dimeric quarternary structure, where C-terminal catalytic domains interact to form an isologous dimer.<ref name="Koch"/> |
== Cofactors == | == Cofactors == | ||
| - | For isoprene synthesis, several mechanisms and metal-binding motifs play an essential role. <scene name='68/686749/Metal_binding_motif/ | + | For isoprene synthesis, several mechanisms and metal-binding motifs play an essential role. <scene name='68/686749/Metal_binding_motif/2'>Metal-binding motifs</scene> were found to be conserved like the “aspartate-rich” motif D345DXXD. These metal ions like Mg2+ or Mn2+ are essential for DMAPP diphosphate to be released. |
PcISPS in fact is the first terpenoid synthase to show up metal binding motifs of terpenoid cyclases. These metal binding motifs have the ability to interact with a trinuclear Mg2+ cluster in complex with DMASPP. Mg2+A binds fully while B and C bind less. This can be considered to occur because of structural geometry in this binding being less good. | PcISPS in fact is the first terpenoid synthase to show up metal binding motifs of terpenoid cyclases. These metal binding motifs have the ability to interact with a trinuclear Mg2+ cluster in complex with DMASPP. Mg2+A binds fully while B and C bind less. This can be considered to occur because of structural geometry in this binding being less good. | ||
| - | The PcISPS-DMASPP complex does not show significant conformational changes in regard to the single PcISPS. In addition to interactions with metal ions, the diphosphate group also accepts hydrogen bonds from R486 and N489. One (monomer A) or two (monomer B) oxygen atoms and D345 also coordinate Mg2+B and Mg2+A. | + | The PcISPS-DMASPP complex does not show significant conformational changes in regard to the single PcISPS. In addition to interactions with metal ions, the diphosphate group also accepts <scene name='68/686749/Hydrogen_bonds/1'>hydrogen bonds</scene> from R486 and N489. One (monomer A) or two (monomer B) oxygen atoms and <scene name='68/686749/Mg-coordination/1'>D345</scene> also coordinate Mg2+B and Mg2+A.<ref name="Koch"/> |
== Biological Relevance == | == Biological Relevance == | ||
| - | The interest in the isoprene synthase and especially the mechanism of this enzyme is based on the aim to develop carbon fuels in bioreactors that do not rest upon a petrochemical process. Isoprene can make up a somehow “greener” source for rubber and plastic products. Furthermore there is a structure-based engineering of terpenoid synthase function to make it accessible to a huge amount of biotechnological applications which are named above. | + | The interest in the isoprene synthase and especially the mechanism of this enzyme is based on the aim to develop carbon fuels in bioreactors that do not rest upon a petrochemical process. Isoprene can make up a somehow “greener” source for rubber and plastic products.<ref name="Koch"/> Furthermore there is a structure-based engineering of terpenoid synthase function to make it accessible to a huge amount of biotechnological applications which are named above.<ref name="Andrea"> PMID:21387007 </ref> |
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Structure of Isoprene Synthase from Grey Poplar Leaves (Populus x canescens)
| |||||||||||