We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
User:Andrea Bauer/Sandbox 956
From Proteopedia
(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 11: | Line 11: | ||
== Description == | == Description == | ||
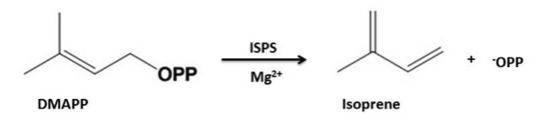
| - | Isoprene Synthase is supposed to be a dimeric enzyme which consists of 595 amino acids and has a molecular mass of 68,386 Da. The protein is made up of alpha-helices which form two alpha-helical domains.<ref name | + | Isoprene Synthase is supposed to be a dimeric enzyme which consists of 595 amino acids and has a molecular mass of 68,386 Da. The protein is made up of alpha-helices which form two alpha-helical domains.<ref name="Koch">PMID:20624401</ref> |
The <scene name='68/686749/N-terminal_domain/1'>N-terminal domain</scene> of the protein chain is folded similar to class II terpenoid synthases that are made up of (𝛼𝛼)6 barrels <ref name="Went">PMID:9519404</ref>. | The <scene name='68/686749/N-terminal_domain/1'>N-terminal domain</scene> of the protein chain is folded similar to class II terpenoid synthases that are made up of (𝛼𝛼)6 barrels <ref name="Went">PMID:9519404</ref>. | ||
Up to now there is no catalytic activity known for this domain. Quite the contrary regarding the <scene name='68/686749/C-terminal_domain/1'>C-terminal domain</scene> of the isoprene synthase: This domain shows up an 𝛼-helical class I terpenoid synthase fold and contains the active site which is surrounded by five 𝛼-helices. The active site of the enzyme is located in a deep hydrophobic pocket which ensures a protection of the reaction intermediate from water.<ref name="Koch"/> | Up to now there is no catalytic activity known for this domain. Quite the contrary regarding the <scene name='68/686749/C-terminal_domain/1'>C-terminal domain</scene> of the isoprene synthase: This domain shows up an 𝛼-helical class I terpenoid synthase fold and contains the active site which is surrounded by five 𝛼-helices. The active site of the enzyme is located in a deep hydrophobic pocket which ensures a protection of the reaction intermediate from water.<ref name="Koch"/> | ||
Current revision
Structure of Isoprene Synthase from Grey Poplar Leaves (Populus x canescens)
| |||||||||||