We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 970
From Proteopedia
(Difference between revisions)
| (4 intermediate revisions not shown.) | |||
| Line 25: | Line 25: | ||
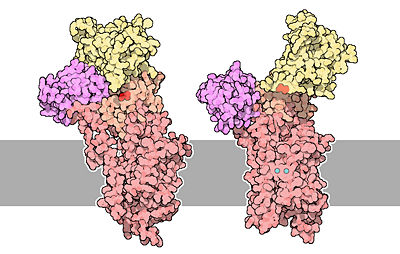
[[Image:51-TheCalciumPumps-calcium-pumps.jpg|400px|center|]] | [[Image:51-TheCalciumPumps-calcium-pumps.jpg|400px|center|]] | ||
| - | The structure on the left is the empty state (free from calcium). Two calcium ions are | + | The structure on the left is the empty state (free from calcium). Two calcium ions are shown in blue and Asp351 is shown in red.<ref name="second"> David Goodsell, 2004 - Calcium pump molecul of the month - PDB, doi: 10.2210/rcsb_pdb/mom_2004_3</ref> |
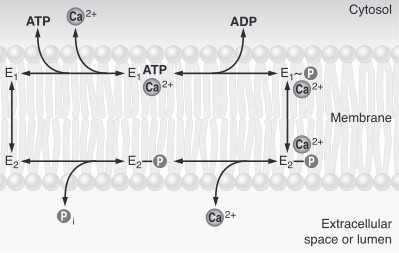
| - | To sum up, calcium pumps have two conformations, E1 and E2. These two conformations are characterized by different specificity for ion binding. When the pump is in the E1 state, it has high calcium affinity and interacts with calcium at one side of the membrane. In the E2 state, the enzyme has a lower calcium affinity and that leads to the release of the ion at the opposite side. E1 has the calcium binding site oriented toward the cytoplasm. E2 has the calcium binding site oriented toward the lumen of the endoplasmic reticulum or toward the extracellular | + | To sum up, calcium pumps have two conformations, E1 and E2. These two conformations are characterized by different specificity for ion binding. When the pump is in the E1 state, it has high calcium affinity and interacts with calcium at one side of the membrane. In the E2 state, the enzyme has a lower calcium affinity and that leads to the release of the ion at the opposite side. E1 has the calcium binding site oriented toward the cytoplasm. E2 has the calcium binding site oriented toward the lumen of the endoplasmic reticulum or toward the extracellular space<ref name="third">Thomas D.Pollard and William C. Earnshaw, - ''Membrane, structure and function'' - Cell Biology (second edition), p.133-136</ref>. The phosphorylated intermediate, E1 can phosphorylate ADP, whereas E2 can only react with water<ref>David H.MacLennan, William J.Rice and N. Michael Green, 1997 - ''The Mechanism of Ca2+ Transport by Sarco(Endo)plasmic Reticulum Ca2+-ATPases'' - The Journal of Biological Chemistry, p.272, 28815-28818, http://www.jbc.org/content/272/46/28815.full.html</ref>. In the absence of calcium, calcium pumps are mainly in E1 state. |
| Line 37: | Line 37: | ||
== Biological Function and Localisation == | == Biological Function and Localisation == | ||
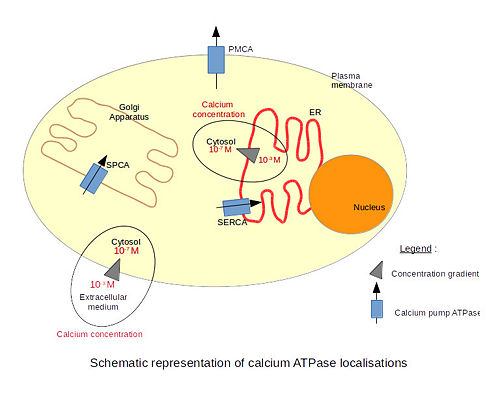
| - | There are 3 types of calcium | + | There are 3 types of calcium ATPases depending on their localization. The '''SERCA''' (Sarco/Endoplasmic Reticulum Calcium ATPase) is localized in the endoplasmic reticulum membranes, including the nuclear envelope. Only the structure of the SERCA has been resolved by x-ray crystallography. The '''PMCA''' (Plasma Membrane Calcium ATPase) is localized in plasma membranes. The '''SPCA''' (Secretory Pathway Calcium ATPase) is localized in the Golgi apparatus membranes. The particularity of this pump is that it is also able to transport Mn2+ ions. |
[[Image:ATPases.jpg|500px|center|]] | [[Image:ATPases.jpg|500px|center|]] | ||
| Line 48: | Line 48: | ||
== Regulations == | == Regulations == | ||
| - | There are different | + | There are different kinds of calcium ATPase regulations. For example, the <scene name='60/604489/Phospholamban/2'>phospholamban</scene> (PLN or PLB) and the <scene name='60/604489/Sarcolipin/1'>sarcolipin</scene> are membrane proteins that regulate the calcium pump in the cardiac muscle and in skeletal muscle cells. These two proteins are close homologous and play the same role. The phospholamban is a phosphoprotein that can be phosphorylated at three distinct sites by various protein kinases (PKA, PKC, CamK...). The phosphorylation state is mediated through beta-adrenergic stimulation. In unphosphorylated state, the phospholamban inhibits the activity of the calcium pump in cardiac and skeletal muscle cells by decreasing the apparent affinity of the ATPase for calcium. The phosphorylation of the protein results in the dissociation of the protein from the ATPase<ref>Marianela G.Dalghi, Marisa M.Fernández, Mariela Ferreira-Gomes, Irene C.Mangialavori, Emilio L.Malchiodi, Emanuel E.Strehler and Juan Pablo F.C.Rossi, 2013 - ''Plasma Membrane Calcium ATPase Activity Is Regulated by Actin Oligomers through Direct Interaction'' - The Journal of Biological Chemistry, p.288, 23380-23393, http://www.jbc.org/content/288/32/23380.full.</ref>. |
| - | Another example of regulation | + | Another example of regulation is the plasma membrane calcium pump carboxy-terminal tail that contains the calmodulin binding domain (regulatory domain) which acts as an auto-inhibitory domain. The binding of the calmodulin frees the pump from autoinhibition<ref>Marisa Brini and Ernesto Carafoli, 2010 - ''The plasma membrane Ca2+ ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium'' - Cold Spring Harbor Perspectives in Biology, http://cshperspectives.cshlp.org/content/3/2/a004168.full</ref>. |
== Dysfunctions and Diseases == | == Dysfunctions and Diseases == | ||
| - | Two human genetic diseases caused by mutations in the SERCA pump have been identified : Brody's disease and Darier's disease.<ref name = "four">Marisa Brini , Ernesto Carafoli, 2009 - ''Calcium pumps in health and disease'' - Physiological Reviews</ref> Brody's disease is caused by 2 mutations in the SERCA1 gene called ATP2A1: <scene name='60/604489/Ile_235/1'>Ile235Asn</scene> and <scene name='60/604489/Glu982/1'>Glu982Lys</scene>. <ref>Nyamkhishig Sambuughin, Elena Zvaritch, Natasha Kraeva, Olga Sizova, Erica Sivak, Kelley Dickson, Margaret Weglinski, John Capacchione, Sheila Muldoon, Sheila Riazi, Susan Hamilton, Barbara Brandom and David H. MacLennan, 2014 - ''Exome analysis identifies Brody myopathy in a family diagnosed with malignant hyperthermia susceptibility'' - Molecular Genetics & Genomic Medicine</ref>. Brody's disease is an autosomal recessive myopathy. The symptoms are muscle stiffness, cramps and progressive impairment of muscle relaxation during exercise. Darier's disease is a rare, dominantly inherited disorder that affects the skin producing a variety of types of | + | Two human genetic diseases caused by mutations in the SERCA pump have been identified : Brody's disease and Darier's disease.<ref name = "four">Marisa Brini , Ernesto Carafoli, 2009 - ''Calcium pumps in health and disease'' - Physiological Reviews</ref> Brody's disease is caused by 2 mutations in the SERCA1 gene called ATP2A1: <scene name='60/604489/Ile_235/1'>Ile235Asn</scene> and <scene name='60/604489/Glu982/1'>Glu982Lys</scene>. <ref>Nyamkhishig Sambuughin, Elena Zvaritch, Natasha Kraeva, Olga Sizova, Erica Sivak, Kelley Dickson, Margaret Weglinski, John Capacchione, Sheila Muldoon, Sheila Riazi, Susan Hamilton, Barbara Brandom and David H. MacLennan, 2014 - ''Exome analysis identifies Brody myopathy in a family diagnosed with malignant hyperthermia susceptibility'' - Molecular Genetics & Genomic Medicine</ref>. Brody's disease is an autosomal recessive myopathy. The symptoms are muscle stiffness, cramps and progressive impairment of muscle relaxation during exercise. Darier's disease is a rare, dominantly inherited disorder that affects the skin producing a variety of types of lesions. It is caused by mutations in the SERCA2 gene (ATP2A2). Defects in the activity or expression level of the pump can cause other dysfunctions such as heart failure or cancer. |
</StructureSection> | </StructureSection> | ||
Current revision
Calcium ATPase
| |||||||||||
References
- ↑ 1.0 1.1 Benjamin Lewin, 2007 - Cells - Jones & Bartlett Learning
- ↑ 2.0 2.1 2.2 Marisa Brini , Ernesto Carafoli, 2009 - Calcium pumps in health and disease - Physiological Reviews
- ↑ David Goodsell, 2004 - Calcium pump molecul of the month - PDB, doi: 10.2210/rcsb_pdb/mom_2004_3
- ↑ Thomas D.Pollard and William C. Earnshaw, - Membrane, structure and function - Cell Biology (second edition), p.133-136
- ↑ David H.MacLennan, William J.Rice and N. Michael Green, 1997 - The Mechanism of Ca2+ Transport by Sarco(Endo)plasmic Reticulum Ca2+-ATPases - The Journal of Biological Chemistry, p.272, 28815-28818, http://www.jbc.org/content/272/46/28815.full.html
- ↑ Marianela G.Dalghi, Marisa M.Fernández, Mariela Ferreira-Gomes, Irene C.Mangialavori, Emilio L.Malchiodi, Emanuel E.Strehler and Juan Pablo F.C.Rossi, 2013 - Plasma Membrane Calcium ATPase Activity Is Regulated by Actin Oligomers through Direct Interaction - The Journal of Biological Chemistry, p.288, 23380-23393, http://www.jbc.org/content/288/32/23380.full.
- ↑ Marisa Brini and Ernesto Carafoli, 2010 - The plasma membrane Ca2+ ATPase and the Plasma Membrane Sodium Calcium Exchanger Cooperate in the Regulation of Cell Calcium - Cold Spring Harbor Perspectives in Biology, http://cshperspectives.cshlp.org/content/3/2/a004168.full
- ↑ Nyamkhishig Sambuughin, Elena Zvaritch, Natasha Kraeva, Olga Sizova, Erica Sivak, Kelley Dickson, Margaret Weglinski, John Capacchione, Sheila Muldoon, Sheila Riazi, Susan Hamilton, Barbara Brandom and David H. MacLennan, 2014 - Exome analysis identifies Brody myopathy in a family diagnosed with malignant hyperthermia susceptibility - Molecular Genetics & Genomic Medicine