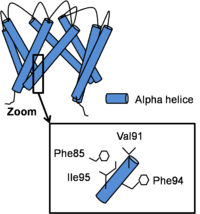We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox Reserved 969
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 40: | Line 40: | ||
====Open Conformation==== | ====Open Conformation==== | ||
| - | Channel opening is a '''conserved mechanism'''.The inner helix '''twist''' and '''bend''' thanks to a<scene name='60/604488/Glycine_conserved/2'>conserved glycine residue </scene>which is considered as the '''gating hinge'''. After this bending, the inner helices twist of 45° around their helical helix and the outer helix tilt tangentially in the same direction by 11° without any twisting motion. As all of helix twist or move inside of a subunit, intra-subunit interactions between inner and outer helix don’t differ a lot. On the contrary, inter-subunit interactions between neighboring inner helix change. In fact, Phe92 swings away and points its side chain towards the central ion conduction pathway due to inner helix bending and the hydrophobic patch slides along the neighboring inner helix by two helical turns and forms new Van der Waals contacts with <scene name='60/604488/Phe85/3'>Phe 85</scene>. This resulted in a '''disruption of the bundle crossing''' and so intra- and inter- subunits interactions in the open state become less important than in the close state. <ref>PMID: 19098917</ref> | + | Channel opening is a '''conserved mechanism'''.The inner helix '''twist''' and '''bend''' thanks to a <scene name='60/604488/Glycine_conserved/2'>conserved glycine residue </scene>which is considered as the '''gating hinge'''. After this bending, the inner helices twist of 45° around their helical helix and the outer helix tilt tangentially in the same direction by 11° without any twisting motion. As all of helix twist or move inside of a subunit, intra-subunit interactions between inner and outer helix don’t differ a lot. On the contrary, inter-subunit interactions between neighboring inner helix change. In fact, Phe92 swings away and points its side chain towards the central ion conduction pathway due to inner helix bending and the hydrophobic patch slides along the neighboring inner helix by two helical turns and forms new Van der Waals contacts with <scene name='60/604488/Phe85/3'>Phe 85</scene>. This resulted in a '''disruption of the bundle crossing''' and so intra- and inter- subunits interactions in the open state become less important than in the close state. <ref>PMID: 19098917</ref> |
| Line 66: | Line 66: | ||
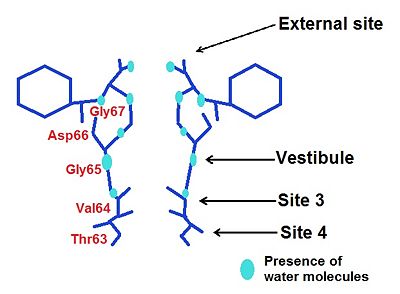
=== Vestibule === | === Vestibule === | ||
| - | In the case of the vestibule, there are too four carbonyl oxygen atom which brings by a valine (Val64). For instance, Na+ is neared to the ligand by this way: distance Na+-ligand=2,9 Ä. Moreover, ions are partially hydrated by four water molecules( they are along with the carboxyl oxygene atoms) : distance ions-H2O=4 Ä. The presence of water allows a greater flexibility in the ion binding so the vestibule may adapt to monovalent cations such as Na+, K+ and Rb+. However, this structure has a greater selectivity for K+ than Na+ : water molecules help to create a selectivity filter thanks to ligand geometry: octahedral arrangement which is impossible with Na+ because of a smaller radius and a hydratation by 5-6 molecules of water <ref> PMID: 16875774 </ref>. | + | In the case of the vestibule, there are too four carbonyl oxygen atom which brings by a valine (Val64). For instance, Na+ is neared to the ligand by this way: distance Na+-ligand=2,9 Ä. Moreover, ions are partially hydrated by four water molecules (they are along with the carboxyl oxygene atoms) : distance ions-H2O=4 Ä. The presence of water allows a greater flexibility in the ion binding so the vestibule may adapt to monovalent cations such as Na+, K+ and Rb+. However, this structure has a greater selectivity for K+ than Na+ : water molecules help to create a selectivity filter thanks to ligand geometry: octahedral arrangement which is impossible with Na+ because of a smaller radius and a hydratation by 5-6 molecules of water <ref> PMID: 16875774 </ref>. |
The nature of the ligands is '''carbonyl-water'''. | The nature of the ligands is '''carbonyl-water'''. | ||
| Line 75: | Line 75: | ||
Moreover we may underscore a higher affinity for K+ than Na+ because of several reason : | Moreover we may underscore a higher affinity for K+ than Na+ because of several reason : | ||
| - | First, we can find 4 | + | First, we can find 4 backbones carbonyl oxygen from <scene name='60/604488/Val64/1'>Val64</scene> which participate in K+ and Rb+ ions chelation because of the formation of an octahedral ligand: an octahedral arrangement oxygen ligands in the channel pore is more favorable for K+ than Na+. |
The lack of selectivity is due to the fact that the NaK channel have an almost identical structure when it is in complex with Na+, K+ or Rb+ : there is no big rearrangement in the structure of the protein depending on the bound ion. So the structure is stable with any ions, so it is non selective. Moreover, it could have a heavy atom contamination but it happens in a smaller extent with K+ than with Na+. | The lack of selectivity is due to the fact that the NaK channel have an almost identical structure when it is in complex with Na+, K+ or Rb+ : there is no big rearrangement in the structure of the protein depending on the bound ion. So the structure is stable with any ions, so it is non selective. Moreover, it could have a heavy atom contamination but it happens in a smaller extent with K+ than with Na+. | ||
Current revision
NaK Channel 3E83
| |||||||||||