Mycobacterium tuberculosis ArfA Rv0899
From Proteopedia
(Difference between revisions)
| (136 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='2l26' size='350' side='right' caption='NMR structure of uncharacterized protein Rv0899 (PDB code [[2l26]])' scene=''> | + | <StructureSection load='2l26' size='350' side='right' caption='NMR structure of uncharacterized protein Rv0899 (PDB code [[2l26]])' scene='61/612805/N-c_rainbow/1'> |
| + | |||
| + | ==Introduction== | ||
| + | The Rv0899 ArfA protein [[http://www.uniprot.org/uniprot/P9WIU5 ARFA_MYCTU]] from [http://en.wikipedia.org/wiki/Mycobacterium_tuberculosis Mycobacterium tuberculosis] H37Rv belongs to the OmpA (outer membrane protein A) family of outer membrane proteins and has been proposed to act as an outer membrane [[porin]] with ion channel activity and contributes to the bacterium's adaptation to the acidic environment in phagosome [http://en.wikipedia.org/wiki/Phagosome] during infection <ref>PMID: 21117233 </ref>. Rv0899 protein encoded by an ammonia release facilator operon that is necessary for rapid ammonia secretion, pH neutralization and adaptation to acidic environments in vitro. Two ''Mycobacterium tuberculosis'' H37Rv genes (Rv0900 [[http://www.uniprot.org/uniprot/P9WJG7 ARFB_MYCTU]] and Rv0901 [[http://www.uniprot.org/uniprot/P9WJG5 ARFC_MYCTU]] ) adjacent to Rv0899 also encode putative membrane proteins, and are found exclusively in association with Rv0899 in the same pathogenic mycobacteria, suggesting that the three may constitute an operon dedicated to a common function. | ||
| + | Asparagine is the primary ammonia source for ''Mycobacterium tuberculosis'' H37Rv at acidic pH <ref>PMID: 21410778 </ref>. | ||
| + | The deletion of this gene impairs the uptake of some water-soluble substances, such as serine, glucose and glycerol <ref>PMID: 12366842 </ref>. | ||
== Structural highlights == | == Structural highlights == | ||
| - | <table><tr><td colspan='2'>[[2l26]] is a 1 chain structure with sequence from | + | <table><tr><td colspan='2'>[[2l26]] is a 1 chain structure with sequence from ''Mycobacterium tuberculosis''. Full experimental information is available from [http://oca.weizmann.ac.il/oca-bin/ocashort?id=2L26 OCA]. For a <b>guided tour on the structure components</b> use [http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2L26 FirstGlance]. |
| - | </td></tr><tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2l26 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2l26 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=2l26 RCSB], [http://www.ebi.ac.uk/pdbsum/2l26 PDBsum]</span></td></tr> | + | <br></td></tr><tr id='resources'><td class="sblockLbl"><b>Resources:</b></td><td class="sblockDat"><span class='plainlinks'>[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=2l26 FirstGlance], [http://oca.weizmann.ac.il/oca-bin/ocaids?id=2l26 OCA], [http://www.rcsb.org/pdb/explore.do?structureId=2l26 RCSB], [http://www.ebi.ac.uk/pdbsum/2l26 PDBsum]</span></td></tr> |
</table> | </table> | ||
<div style="background-color:#fffaf0;"> | <div style="background-color:#fffaf0;"> | ||
==Structure Section== | ==Structure Section== | ||
| - | The 326-residue Rv0899 ArfA | + | The 326-residue Rv0899 ArfA contains three domains: an N-terminal domain (M domain) (residues 1-72) which includes a sequence of 20 hydrophobic amino acids required for membrane translocation. |
[[Image:N-ter domain.jpg|150px]] | [[Image:N-ter domain.jpg|150px]] | ||
| Line 14: | Line 19: | ||
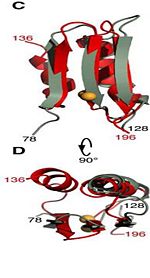
Residues <scene name='61/612805/N-c_rainbow/1'>73-326</scene> form a mixed alpha/beta-globular structure, encompassing two independently folded modules corresponding to the B and C domains connected by a flexible linker. | Residues <scene name='61/612805/N-c_rainbow/1'>73-326</scene> form a mixed alpha/beta-globular structure, encompassing two independently folded modules corresponding to the B and C domains connected by a flexible linker. | ||
| - | The central B domain | + | The central B domain <scene name='61/612805/Secondary_structure_b/1'>residues 73-200</scene> folds with three parallel/antiparallel {{Template:ColorKey_Helix}} packed against six parallel/antiparallel {{Template:ColorKey_Strand}} that form a flat beta-sheet. |
| - | + | <scene name='61/612805/Surface2/1'>The core</scene> is {{Template:ColorKey_Hydrophobic}}, while the exterior is {{Template:ColorKey_Polar}} and predominantly acidic. The two subdomains are symmetric about α2, and their backbone atoms can be aligned with a RMSD [http://http://en.wikipedia.org/wiki/Root-mean-square_deviation] of 1.8Å, by performing a 180° rotation of either one around an axis normal to the 6-stranded β-sheet. | |
| - | + | ||
| - | + | ||
| - | The | + | [[Image:180 rotation BON.jpg|150px]] |
| - | = Function == | ||
| - | The membrane protein Rv0899 ArfA belongs to the OmpA (outer membrane protein A) family of outer membrane proteins and has been proposed to act as an outer membrane [[porin]] and to contribute to the bacterium's adaptation to the acidic environment of the phagosome[http://en.wikipedia.org/wiki/Phagosome] during infection.The deletion of this gene impairs the uptake of some water-soluble substances, such as serine, glucose, and glycerol. | ||
| - | Using [[NMR]] chemical shift perturbation and isothermal calorimetric titration assays, Rv0899 was able to interact with <scene name='61/612805/Binding-site_for_zn/1'>Zn(2+) ions</scene>, which may indicate a role for Rv0899 in the process of Zn(2+) acquisition. | ||
| - | <ref>PMID: 22108166 </ref> | ||
| - | ''Mycobacterium tuberculosis'' ArfA (Rv0899) is a membrane protein encoded by an ammonia release facilator operon that is necessary for rapid ammonia secretion, pH neutralization and adaptation to acidic environments in vitro. Its C-terminal domain (C domain) shares significant sequence homology with the OmpA-like family of peptidoglycan-binding domains, suggesting that its physiological function in acid stress protection may be related to its interaction with the mycobacterial cell wall. It exhibits pH-dependent conformational dynamics (with significant heterogeneity at neutral pH and a more ordered structure at acidic pH), which could be related to its acid stress response. The C domain associates tightly with polymeric peptidoglycan isolated from ''Mycobacterium tuberculosis''. Its functions in acid stress protection and <scene name='61/612805/The_peptidoglycan_binding_site/1'>peptidoglycan binding</scene> suggest a link between the acid stress response and the physicochemical properties of the mycobacterial cell wall.<ref>PMID: 22206986 </ref>. | ||
| - | + | The C domain <scene name='61/612805/Secondary_structure_c/1'>residues 201-326</scene> The C domain of wild-type ArfA <scene name='61/612805/C_domain/3'></scene> <scene name='61/612805/C_domain_1/1'> folds </scene> into four {{Template:ColorKey_Strand}} and four {{Template:ColorKey_Helix}}.Three parallel (β1, β2, β3) and one antiparallel (β4) β-strands form a four-stranded β-sheet (β1–β4-β2–β3) that packs against three α-helices (α1, α2, α3), while a fourth helix (α4) extends from the N-terminus of β4. The structure <scene name='61/612805/C_domain_stabilization/2'> is stabilized </scene> by disulfide bond between C208 and C250, by a network of hydrophobic contacts between α1, α2 and β4 (L211, I215, V243, L247, Ile323 and V325) between side chains and by a hydrogen bond between the backbone amide of V325 and the side-chain carbonyl of Q212. | |
| + | ==Function== | ||
| + | |||
| + | Stress response of the bacterium and adaptation to the acidic external environment. | ||
| - | + | 1. Acquisition of Zn(2+) ions by {{Template:ColorKey Composition Ligand}} <scene name='61/612805/Binding-site_for_zn/1'>binding-site L82, D96, F97, H125, D127 and V129</scene> for DNA replication and transcription <ref>PMID: 22108166 </ref>. | |
| - | + | ||
| - | + | ||
| + | 2. Rapid ammonia secretion to external environment: | ||
| + | deamidation of the amino acid pair <scene name='61/612805/Asn111_and_gly112/1'> Asn111-Gly112 </scene>, located at the end of α1 and preceding L3, a pH-dependent reaction whereby Asn is converted to Asp and ammonia is released. Asparagine residues preceding glycine, and situated in conformationally flexible regions of proteins, are frequently deamidated, with potentially significant consequences for protein regulation and function <ref>PMID: 20199110</ref>. [[Image:Asparaginase-reaction.jpg|250px]] | ||
| - | + | 3. Stabilization of the outer membrane: | |
| + | 1. pH-dependent conformational dynamics of hydrophobic cluster of L232, F225, L240, A244, V281, L285 <scene name='61/612805/D236_before_mutation/1'>in neutral pH (D236) </scene> that folds to a more ordered structure like a flap at <scene name='61/612805/D236a_after_mut/3'>acidic pH (D236A) </scene>. | ||
| - | == Relevance == | ||
| - | Probably plays a role in ammonia secretion that neutralizes the medium at pH 5.5,and preceded exponential growth of ''Mycobacterium tuberculosis'', although it does not play a direct role in ammonia transport.[[http://www.uniprot.org/uniprot/P9WIU5 ARFA_MYCTU]]. | ||
| - | [[Image:Bdomain.jpg|150px]] | ||
| + | 2. Binding to peptidoglycan and phospholipid layer. | ||
| + | a) The B domain has homology with conserved putative <scene name='61/612805/Conserved_g95_and_g164_in_bon/2'>lipid-binding BON</scene> (bacterial OsmY and nodulation) superfamily domains and conserved Gly95 and Gly164 [http://www.ebi.ac.uk/interpro/entry/IPR014004], which putative functions are prevention of shrinkage of inner and outer membrane by binding to the phospholipid layer, nitrogen fixation and / or nitrogen metabolism <ref>PMID: 12878000 </ref>. | ||
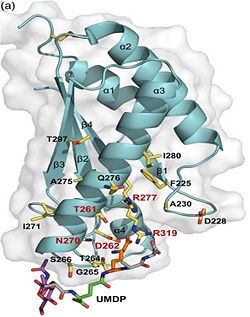
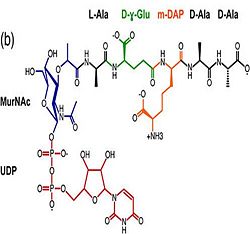
| - | <scene name='61/612805/ | + | b)The C domain has homology to the OmpA-C-like superfamily of periplasmic peptidoglycan-binding sequences, found in several types of bacterial membrane proteins.Contribution for structural strength to the bacterial cell wall under acid or other stress conditionsIts functions by binding to peptidoglycan biosyntheesis intermediate uridine-5-'-diphosphate-MurNAc–L-Ala–D-γ-Glu–m-DAP–D-Ala–D-Ala (UMDP) [http://en.wikipedia.org/wiki/Peptidoglycan] binding site. <scene name='61/612805/Peptidoglycan_binding_site/1'>(R277, R319, T261, D262, N270)</scene>. These residues are strictly conserved in the OmpA -like family. The side chain of m-DAP is stabilized by charge-charge interactions between its electronegative carbonyl group with R277 and R319 guanidinium groups and with the N270 carboxamide and between its electropositive amino group with the D262 carboxyl and the T261 hydroxyl. UMDP could interact through contacts of its γ-Glu3 and Ala2 backbone amides with the side-chain hydroxyl of S266, of its MurNAc O3 with the amide proton of E267, and of its MurNAc NH2 with the E267 carboxyl <ref>PMID: 22206986 </ref>. |
| + | [[Image:123456.jpg|250px]] [[Image:MurNac11.jpg|250px]] | ||
| - | + | ||
| - | <scene name='61/612805/Hydrophobic_region_of_d236a/1'>Hydrophobic region of mutant ArfA-c (D236A)</scene> | ||
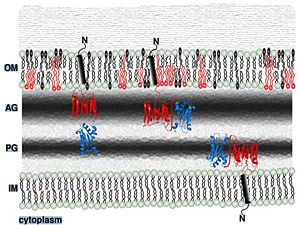
| + | ==Putative localization in the outer membrane== | ||
| + | Periplasmic space | ||
| + | [[Image:Memb localization.jpg|300px]] | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||