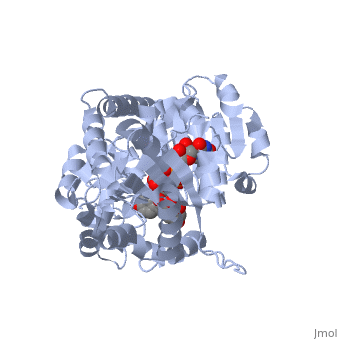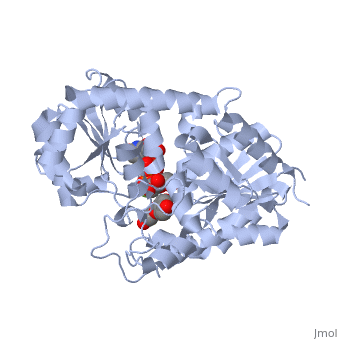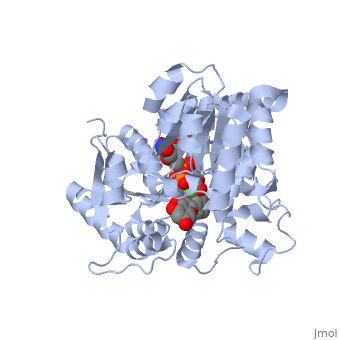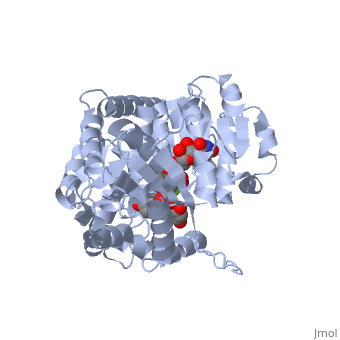
Vitis vinifera Flavonoid 3-O-Glucosyltransferase (Vv3GT)
From Proteopedia
(Difference between revisions)
m (adding pages to the "Pages with quizzes" category) |
|||
| (28 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
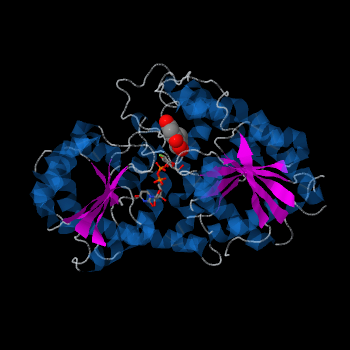
| - | <StructureSection load='2c1z' size='340' side='right' caption=' | + | <StructureSection load='2c1z' size='340' side='right' caption='Flavonoid 3-O-glucosyltransferase complex with UDP-glucose derivative and kaempherol (PDB code [[2c1z]])' scene=''> |
| - | Vitis vinifera Flavonoid 3-O-Glucosyltransferase (Vv3GT) is involved in the modification of grape anthocyanins and thus could affect their color stability. The | + | ''Vitis vinifera'' '''Flavonoid 3-O-Glucosyltransferase''' (Vv3GT) is involved in the modification of grape [http://en.wikipedia.org/wiki/Anthocyanin anthocyanins] (a plant pigment) and thus could affect their water solubility and color stability <ref>PMID:21443631</ref>. The addition of a sugar molecule on the anthocyanin is a preliminary step to its transport to the cell vacuole. The transfer to the vacuole is important for the pigment accumulation. The anthocyanin accumulation plays a significant role in quality of agricultural produce, as it affects fruit color and its health benefits as a natural antioxidant. This enzyme affects the quality of both table grapes and wine grapes. |
== Introduction == | == Introduction == | ||
| - | Vv3GT belongs to Glycosyltransferases (GTs), a large family of enzymes involved in the transfer of sugar residues from a sugar donor to various substrates. Glycosylation of metabolites in plants is usually catalyzed by glycosyltransferases (GTs) belonging to the GT1 sub-family | + | Vv3GT belongs to [http://en.wikipedia.org/wiki/Glycosyltransferase Glycosyltransferases] (GTs), a large family of enzymes involved in the transfer of sugar residues from a sugar donor to various substrates. Glycosylation of metabolites in plants is usually catalyzed by glycosyltransferases (GTs) belonging to the GT1 sub-family, as classified by the [http://www.cazy.org CAZy] database , which use UDP-activated sugars as the major donor molecule and are thus referred to as UGTs. The Glycosyltransferase activity is highly important for the synthesis of thousands of plant metabolites. This enzymes differ in their specificity for substrate, position of glycosylation on the substrate and recognition of sugar donor. These differences are important and could affect the function and stability of the metabolite. |
| + | == Relevance == | ||
| + | |||
| + | A good example for the importance of glycosyltransferase specificity is shown in Citrus <ref>PMID:15361143</ref> . the difference in the position of glycosylation affects fruit flavor. Glycosylation of one position lids to the synthesis of bitter substances while the addition of same molecule at a different position leads to the synthesis of tasteless substances. | ||
| - | == Disease == | ||
| - | == Relevance == | ||
== Structural highlights == | == Structural highlights == | ||
| - | Despite low primary sequence similarity, the secondary and tertiary structures of GTs are highly conserved | + | Despite low primary sequence similarity, the secondary and tertiary structures of GTs are highly conserved <ref>PMID:19217634</ref>. |
=== GT-B === | === GT-B === | ||
| + | Plant glycosyltransferases assume one of two folds, GT-A or GT-B. Vv3GT is a GT-B enzyme<ref>PMID:19217634</ref>. GT-B enzymes consists of two β/α/β Rossmann-like domains <ref>PMID:16482224</ref>. The two domains are associated and face each other with the active-site lying between them. These domains are associated with the donor and acceptor substrate binding sites. | ||
| + | The <scene name='69/692252/2c1z_rainbow/1'>N-terminal and C-terminal domain</scene>, linked by a flexible loop and α-helix with a hinge region. The linker could be important for sugar donor binding. This linker region holds the UDP-sugar donor next to the C-terminal PSPG motif. The C-terminal domain is highly conserved and binds the UDP sugar donor (e. g. UDP-Glc). On the other hand, the N-terminal domain is not conserved it binds the substrate and provides catalytically active amino acids. | ||
| + | <table cellpadding="5" cellspacing="0"> | ||
| + | <tr> | ||
| + | <td bgcolor="#0000ff"><font color="#ffffff" face="sans-serif"><b>N, 5'</b></font></td> | ||
| + | <td bgcolor="#0080ff"> </td> | ||
| + | <td bgcolor="#00c0ff"> </td> | ||
| + | <td bgcolor="#00e0b0"> </td> | ||
| + | <td bgcolor="#50e000"> </td> | ||
| + | <td bgcolor="#a0e000"> </td> | ||
| + | <td bgcolor="#e0e000"> </td> | ||
| + | <td bgcolor="#ffa000"> </td> | ||
| + | <td bgcolor="#ff0000"><font color="#ffffff" face="sans-serif"><b>C, 3'</b></font></td> | ||
| + | </tr></table> | ||
| + | [[Image:2C1Z GT-B b.png|frame|alt=Puzzle globe| Vv3GT with two Rossmann-like domains]] | ||
| + | === PSPG === | ||
| - | + | ||
| - | + | The plant UGTs are characterized by sharing a highly conserved 44 amino acid motif referred to as the PSPG motif (Plant Secondary Product Glycosyltransferase motif). Amino acids of the PSPG motif provide most of the interactions with the sugar donor molecule. 10 highly conserved residues of the 44 amino acid PSPG motif are observed to directly interact with the UDP-sugar. In Vv3GT the <scene name='60/607848/2c1z_pspg/2'>PSPG</scene> motif begins with Pro 334 and ends with Gln 375. | |
| - | + | Three conserved motifs involved in sugar binding are present in Vv3GT. The first, a <scene name='60/607848/2c1z_loop_n5_label/1'>loopN5</scene> motif (Thr 141; Ala 142) involved in sugar binding. The second, a <scene name='69/692252/2c1z_wns_label/2'>WNS</scene> (Trp 353; Asn 354; Ser 355) motif residues are involved in binding UDP phosphates. The third, <scene name='69/692252/2c1z_d_eq_label/1'>D/EQ</scene> motif residues also involved in sugar binding (Asp 374; Gln 375). The WNS and D/EQ motifs are part of the highly conserved PSPG region. | |
| + | == 3D structure of glucosyltransferase == | ||
| - | [[ | + | [[Glycosyltransferase]] |
| - | == | + | == Quiz == |
| - | + | <quiz display=simple> | |
| - | + | {Plant GT1 family members use this molecule as their main sugar donor: | |
| - | < | + | |type="[]"} |
| + | - ATP. | ||
| + | - H2O. | ||
| + | + UDP. | ||
| + | - Flavonoid. | ||
| - | Three conserved motifs involved in sugar binding are present in Vv3GT. The first, a <scene name='60/607848/2c1z_loop_n5_label/1'>loopN5</scene> motif (Thr 141; Ala 142) involved in sugar binding. The second, a <scene name='69/692252/2c1z_wns_label/1'>WNS</scene> (Trp 354; Asn 355; Ser 356) motif residues are involved in binding UDP phosphates. The third, <scene name='69/692252/2c1z_d_eq_label/1'>D/EQ</scene> motif residues also involved in sugar binding (Asp 374; Gln 375). The WNS and D/EQ motifs are part of the highly conserved PSPG region. | ||
| - | == | + | {How many residues are included in the PSPG motif? |
| + | |type="[]"} | ||
| + | - 3. | ||
| + | - 5. | ||
| + | - 10. | ||
| + | + 44. | ||
| + | |||
| + | |||
| + | {How many of the PSPG motif residues directly interact with the UDP-sugar donor? | ||
| + | |type="[]"} | ||
| + | - 3. | ||
| + | - 5. | ||
| + | + 10. | ||
| + | - 44. | ||
| + | |||
| + | |||
| + | {The Rossmann-like domains assume the following secondary and tertiary structures: | ||
| + | |type="[]"} | ||
| + | - Beta berral . | ||
| + | - Only α Helixes. | ||
| + | - Only β sheets. | ||
| + | + β-α-β structure. | ||
| + | |||
| + | |||
| + | {In which aspects can glycosylation affect the nature of a metabolite? | ||
| + | |type="[]"} | ||
| + | - Color. | ||
| + | - Flavor. | ||
| + | - Water solubility . | ||
| + | + All of the above. | ||
| + | {Is Vv3GT one of the most important proteins out there? | ||
| + | |type="[]"} | ||
| + | + Yes. | ||
| + | + No. | ||
| + | + Maybe. | ||
| + | </quiz> | ||
</StructureSection> | </StructureSection> | ||
| Line 45: | Line 101: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
| + | == External links == | ||
| + | |||
| + | *[http://en.wikipedia.org/wiki/Anthocyanin Anthocyanins in WikipediA] | ||
| + | *[http://en.wikipedia.org/wiki/Glycosyltransferase Glycosyltransferases in WikipediA] | ||
| + | *[http://www.cazy.org CAZy database] | ||
| + | |||
| + | [[Category:Pages with quizzes]] | ||
Current revision
| |||||||||||
References
- ↑ Yonekura-Sakakibara K, Hanada K. An evolutionary view of functional diversity in family 1 glycosyltransferases. Plant J. 2011 Apr;66(1):182-93. doi: 10.1111/j.1365-313X.2011.04493.x. PMID:21443631 doi:http://dx.doi.org/10.1111/j.1365-313X.2011.04493.x
- ↑ Frydman A, Weisshaus O, Bar-Peled M, Huhman DV, Sumner LW, Marin FR, Lewinsohn E, Fluhr R, Gressel J, Eyal Y. Citrus fruit bitter flavors: isolation and functional characterization of the gene Cm1,2RhaT encoding a 1,2 rhamnosyltransferase, a key enzyme in the biosynthesis of the bitter flavonoids of citrus. Plant J. 2004 Oct;40(1):88-100. PMID:15361143 doi:http://dx.doi.org/10.1111/j.1365-313X.2004.02193.x
- ↑ Osmani SA, Bak S, Moller BL. Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry. 2009 Feb;70(3):325-47. doi: 10.1016/j.phytochem.2008.12.009. Epub, 2009 Feb 13. PMID:19217634 doi:http://dx.doi.org/10.1016/j.phytochem.2008.12.009
- ↑ Osmani SA, Bak S, Moller BL. Substrate specificity of plant UDP-dependent glycosyltransferases predicted from crystal structures and homology modeling. Phytochemistry. 2009 Feb;70(3):325-47. doi: 10.1016/j.phytochem.2008.12.009. Epub, 2009 Feb 13. PMID:19217634 doi:http://dx.doi.org/10.1016/j.phytochem.2008.12.009
- ↑ Offen W, Martinez-Fleites C, Yang M, Kiat-Lim E, Davis BG, Tarling CA, Ford CM, Bowles DJ, Davies GJ. Structure of a flavonoid glucosyltransferase reveals the basis for plant natural product modification. EMBO J. 2006 Mar 22;25(6):1396-405. Epub 2006 Feb 16. PMID:16482224