RiAFP
From Proteopedia
(Difference between revisions)
m (adding pages to the "Pages with quizzes" category) |
|||
| (31 intermediate revisions not shown.) | |||
| Line 6: | Line 6: | ||
== Overall Structure == | == Overall Structure == | ||
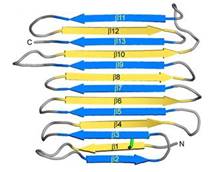
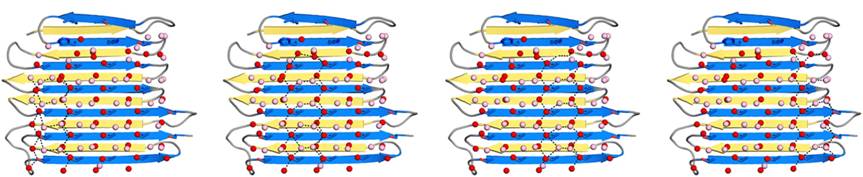
| - | [[Image:Fig2_B.jpg|frame|Fig. 1. Secondary structure diagram]] The crystallographic structure of ''Ri''AFP was defined recently<ref>DOI 10.1074/jbc.M113.450973</ref>. ''Ri''AFP has a novel β-solenoid architecture that forms <scene name='60/607864/Beta_sheets/1'>β-sandwich</scene>. This sandwich is composed of two parallel remarkably regular <scene name='60/607864/Beta_sheets_colored/1'> 6 and 7 stranded-sheets</scene>. The β-sheets lie on top of each other with the upper and lower strands parallel but in the opposite orientation. β1 and β2 strands are untiparallel to the other strands in each sandwich plane. Two ends deviate from β helix regularity by forming <scene name='60/607864/Beta_sheets_capping/2'>capping structures</scene> (Figure 1). These capping structures help to prevent end-to-end associations that would | + | [[Image:Fig2_B.jpg|frame|Fig. 1. Secondary structure diagram]] The crystallographic structure of ''Ri''AFP was defined recently<ref>DOI 10.1074/jbc.M113.450973</ref>. ''Ri''AFP has a novel β-solenoid architecture that forms <scene name='60/607864/Beta_sheets/1'>β-sandwich</scene>. This sandwich is composed of two parallel remarkably regular <scene name='60/607864/Beta_sheets_colored/1'> 6 and 7 stranded-sheets</scene>. The β-sheets lie on top of each other with the upper and lower strands parallel but in the opposite orientation. β1 and β2 strands are untiparallel to the other strands in each sandwich plane. Two ends deviate from β helix regularity by forming <scene name='60/607864/Beta_sheets_capping/2'>capping structures</scene> (Figure 1). These capping structures help to prevent end-to-end associations that would impair the solubility of ''Ri''AFP and lead to oligomerization and aggregation. |
| - | The three residues in β-strand 11 at the C terminus <scene name='60/607864/Gln110_gln112_ile114/1'>(Gln110, Gln112, and Ile114)</scene> that are too bulky to be accommodated into the core may also contribute to the capping structure to prevent amyloid-like polymerization. | + | The three residues in β-strand 11 at the C terminus <scene name='60/607864/Gln110_gln112_ile114/1'>(Gln110, Gln112, and Ile114)</scene> that are too bulky to be accommodated into the core may also contribute to the capping structure to prevent amyloid-like polymerization. ''Ri''AFP solenoid possesses compressed nature with average distance between the sheets of only 6 Å. In the core of ''Ri''AFP, the side chains within apposed β-strands from the two β-sheets are staggered, allowing the side chains to interdigitate and pack tightly against one another. Most of the <scene name='60/607864/Core_structure/1'>side chains</scene> in the core are from <span style="color:pink;background-color:black;font-weight:bold;">Ala</span>, <span style="color:green;background-color:black;font-weight:bold;">Ser</span> and <span style="color:yellow;background-color:black;font-weight:bold;">Thr</span>. Those residues create a more compact fold that may contribute to the high stability and antifreeze activity of ''Ri''AFP. Within the core there are <scene name='60/607864/Hydrogen_bonds/2'>hydrogen bonds</scene> between Thr-Ser (65-55, 85-75, 132-124 respectively) and one <scene name='60/607864/S-s_bond/1'>disulfide bond</scene> between Cys4-Cys21, that contribute to stabilization of the whole structure. The β-turns in the structure contain mostly Gly or Pro residues. |
| - | The <scene name='60/607864/Assymetric_unit/1'>asymmetric unit</scene> (26-kDa) comprises two | + | The <scene name='60/607864/Assymetric_unit/1'>asymmetric unit</scene> (26-kDa) comprises two ''Ri''AFP molecules juxtaposed with their ice-binding surfaces, however the protein is monomer in the solution. |
== Ice Binding Surface (IBS) == | == Ice Binding Surface (IBS) == | ||
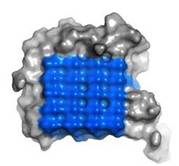
[[Image:Fig4_D.jpg|frame|left|Fig. 2. Ice-binding surface ]] | [[Image:Fig4_D.jpg|frame|left|Fig. 2. Ice-binding surface ]] | ||
| - | IBS of RiAFP contains five expanded <scene name='60/607864/Ibs/1'>TXTXTXT motifs</scene> within the top β–sheet. These motifs are remarkably regular, allowing any rows/columns of TXTXTTX motifs to be exactly superposed onto any other rows/columns. Threonine residuses are crucial for maintaining antifreeze activity. It was found in other AFPs that mutations of the Thrs within these motifs decrease the | + | IBS of RiAFP contains five expanded <scene name='60/607864/Ibs/1'>TXTXTXT motifs</scene> within the top β–sheet. These motifs are remarkably regular, allowing any rows/columns of TXTXTTX motifs to be exactly superposed onto any other rows/columns. Threonine residuses are crucial for maintaining antifreeze activity. It was found in other AFPs that mutations of the Thrs within these motifs decrease the TH. The Thr hydroxyls define a large flat IBS of 420 Å2, which correlates with high antifreeze activity (Figure 2). |
| Line 24: | Line 24: | ||
== Molecular Basis for Ice Binding == | == Molecular Basis for Ice Binding == | ||
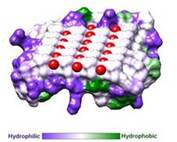
| - | IBS is less hydrophilic than the other β–sheet, which is consistent with its role of interacting with the ice ( | + | IBS is less hydrophilic than the other β–sheet, which is consistent with its role of interacting with the ice (Figure 3). Adsorption of the AFP ice-binding surface to ice is facilitated by the flatness of the IBS of the ''Ri''AFP. The Sc (shape complementarity) values between ''Ri''AFP and ice interfaces range from 0.75-0.78, where 1.0 indicates perfect match. For comparison, antigen-antibody complexes usually have their Sc values in the range of 0.64–0.68. |
| - | Thr hydroxyls bind 3 ranks of 6 <scene name='60/607864/Isosurface/ | + | Thr hydroxyls bind 3 ranks of 6 <scene name='60/607864/Isosurface/6'>water molecules</scene> with equivalent spacing between the 4 ranks of Thr side chains. These water molecules are bound tightly, they have lost both translational and rotational freedom and resemble those in an ice lattice. The waters observed in the computational simulation appear to be organized in an ice-like formation, with close matches to the primary prism (Figure 4) and basal planes of ice. IBS is responsible for ordering an ice-like array of anchored “clathrate” water molecules to promote adsorption to ice. |
{|style="margin: 0 auto;" | {|style="margin: 0 auto;" | ||
| Line 33: | Line 33: | ||
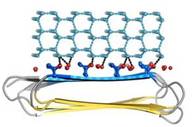
| - | An advantage of this indirect binding mechanism is that the organized waters are still fluid enough to make flexible matches to the ice-like quasi-liquid layer around the ice before becoming rigidified as the junction layer freezes. Alignment of IBS of | + | An advantage of this indirect binding mechanism is that the organized waters are still fluid enough to make flexible matches to the ice-like quasi-liquid layer around the ice before becoming rigidified as the junction layer freezes. Alignment of IBS of ''Ri''AFP to hexagonal ice showed possible interactions of the protein with the several ice planes. Several ranks of matching Thr hydroxyls to water molecules give the ''Ri''AFP an ability to bind multiple ice planes, by that providing the protein hyperactivity (Figure 5). |
| - | [[Image:Fig7_a.jpg|frame|Fig. 5. ]] | + | [[Image:Fig7_a.jpg|frame|Fig. 5. Four water patterns, that could potentially independently bind the ''Ri''AFP to ice.Crystallographic waters are in red; simulation waters are in pink; dashed lines (black) represent potential hydrogen bonds]] |
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | == '''Quiz''' == | ||
| + | <quiz display=simple> | ||
| + | |||
| + | {This page is about ''Ri''AFP, which is a distinct type of AFPs. AFP stands for : | ||
| + | |type="()"} | ||
| + | - Active Fire Protection | ||
| + | + Antifreeze Protein | ||
| + | - Atypical Facial Pain | ||
| + | - Armed Forces of the Philippines | ||
| + | |||
| + | {''Ri''AFP is produced by : | ||
| + | |type="()"} | ||
| + | - spruce budworm ''Choristoneura fumiferana'' | ||
| + | - mealworm ''Tenebrio Molitor'' | ||
| + | + longhorn beetle ''Rhagium inquisitor'' | ||
| + | - antarctic bacterium ''Microdera punctipennis'' | ||
| + | |||
| + | {''Ri''AFP has of the highest antifreeze activities measured for any AFP. | ||
| + | |type="()"} | ||
| + | + true | ||
| + | - false | ||
| + | |||
| + | { | ||
| + | |type="{}"} | ||
| + | What is the molecular weight of ''Ri''AFP monomer? | ||
| + | { 13-kDa|13 kDa } | ||
| + | |||
| + | {AFPs inhibit ice crystals growth through the: | ||
| + | |type="()"} | ||
| + | - adsorption inhibition model by colligative depression of the freezing point | ||
| + | + adsorption inhibition model by noncolligative depression of the freezing point | ||
| + | - Gibbs-Thompson-Herring (i.e. Kelvin) effect by increasing the melting point of the solution | ||
| + | - Gibbs-Thompson-Herring (i.e. Kelvin) effect by maintaining ice front flat | ||
| + | |||
| + | {What is ''Ri''AFP architecture? | ||
| + | |type="()"} | ||
| + | - α domains structure | ||
| + | - α/β barrel | ||
| + | - four-helix bundle | ||
| + | + β solenoid | ||
| + | |||
| + | { | ||
| + | |type="{}"} | ||
| + | How many β sheets do ''Ri''AFP have? | ||
| + | { 13 } | ||
| + | |||
| + | {What residues are crucial for maintaining antifreeze activity? | ||
| + | |type="()"} | ||
| + | + Threonine | ||
| + | - Alanine | ||
| + | - Serine | ||
| + | - Glutamine | ||
| + | - Isoleucine | ||
| + | |||
| + | {''Ri''AFP has two disulfide bonds. | ||
| + | |type="()"} | ||
| + | - true | ||
| + | + false | ||
| + | |||
| + | {''Ri''AFP binds ice crystals: | ||
| + | |type="()"} | ||
| + | - covalently | ||
| + | + via anchored “clathrate” water molecules | ||
| + | - directly | ||
| + | - through van der Waals' interactions | ||
</StructureSection> | </StructureSection> | ||
| Line 47: | Line 130: | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | |||
| + | [[Category:Pages with quizzes]] | ||
Current revision
| |||||||||||
3D structures of antifreeze protein
References
- ↑ Jia Z, Davies PL. Antifreeze proteins: an unusual receptor-ligand interaction. Trends Biochem Sci. 2002 Feb;27(2):101-6. PMID:11852248
- ↑ Chantelle J. Capicciotti, Malay Doshi and Robert N. Ben (2013). Ice Recrystallization Inhibitors: From Biological Antifreezes to Small Molecules, Recent Developments in the Study of Recrystallization, Prof. Peter Wilson (Ed.), ISBN: 978-953-51-0962-4, InTech doi:http://dx.doi.org/10.5772/54992
- ↑ Drori R, Celik Y, Davies PL, Braslavsky I. Ice-binding proteins that accumulate on different ice crystal planes produce distinct thermal hysteresis dynamics. J R Soc Interface. 2014 Sep 6;11(98):20140526. doi: 10.1098/rsif.2014.0526. PMID:25008081 doi:http://dx.doi.org/10.1098/rsif.2014.0526
- ↑ Raymond JA, DeVries AL. Adsorption inhibition as a mechanism of freezing resistance in polar fishes. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2589-93. PMID:267952
- ↑ Hakim A, Nguyen JB, Basu K, Zhu DF, Thakral D, Davies PL, Isaacs FJ, Modis Y, Meng W. Crystal structure of an insect antifreeze protein and its implications for ice binding. J Biol Chem. 2013 Apr 26;288(17):12295-304. doi: 10.1074/jbc.M113.450973. Epub, 2013 Mar 12. PMID:23486477 doi:10.1074/jbc.M113.450973