Human ABO(H) Blood Group Glycosyltransferases
From Proteopedia
(Difference between revisions)
| (5 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
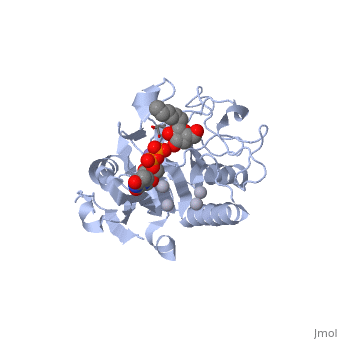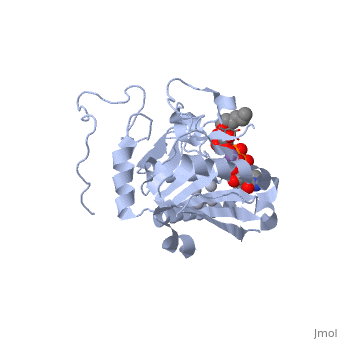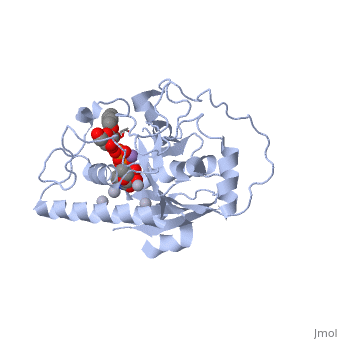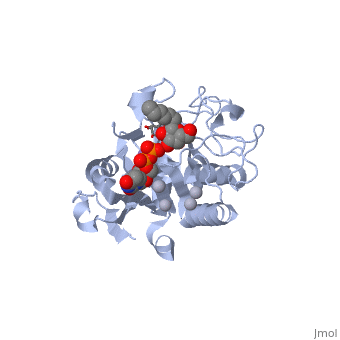
| - | <StructureSection load='1lzj' size='340' side='right' caption=' | + | <StructureSection load='1lzj' size='340' side='right' caption='GTB + UDP + H antigen complex with Hg+2 (grey) and Mn+2 (purple) ions (PDB code [[1lzj]])' scene=''> |
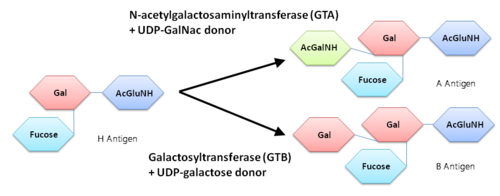
Human ABO(H) blood group glycosyltransferases GTA and GTB catalyze the final monosaccharide addition in the biosynthesis of the human A and B blood group antigens. | Human ABO(H) blood group glycosyltransferases GTA and GTB catalyze the final monosaccharide addition in the biosynthesis of the human A and B blood group antigens. | ||
| Line 22: | Line 22: | ||
'''General topology of GTA and GTB''' | '''General topology of GTA and GTB''' | ||
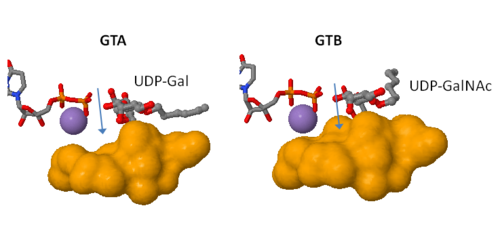
| - | Both GTs consist of 354 residues and they only differ by four "critical" amino acids. | + | Both GTs consist of 354 residues and they only differ by four "critical" amino acids<ref> Patenaude SI, Seto NO, Borisova SN, Szpacenko A, Marcus SL, Palcic MM, Evans SV. The structural basis for specificity in human ABO(H) blood group biosynthesis. Nat Struct Biol. 2002 Sep;9(9):685-90. PMID:12198488 doi:http://dx.doi.org/10.1038/nsb832</ref>. |
the polypeptide chain is organized in <scene name='69/691577/Nandc_terminal/1'>two domains</scene> separated by a cleft, approximately 13 Å wide, containing the active site which consist all four critical amino acid residues. The N-terminal (blue), includes a [http://en.wikipedia.org/wiki/Rossmann_fold Rossmann fold] and recognizes the nucleotide donor, whereas the disaccharide acceptor binding site is formed by residues in the C-terminal domain (green) in combination with bound UDP. | the polypeptide chain is organized in <scene name='69/691577/Nandc_terminal/1'>two domains</scene> separated by a cleft, approximately 13 Å wide, containing the active site which consist all four critical amino acid residues. The N-terminal (blue), includes a [http://en.wikipedia.org/wiki/Rossmann_fold Rossmann fold] and recognizes the nucleotide donor, whereas the disaccharide acceptor binding site is formed by residues in the C-terminal domain (green) in combination with bound UDP. | ||
In the middle of the cleft located a <scene name='69/691577/Dxd_mn2/1'>DXD motif</scene>, highly conserved in a large number of glycosyltransferases, which coordinates the Mn2+ ion and was suggested to have a role in catalysis<ref>Busch, C. et al. J. Biol. Chem. 273, 19566−19572 (1998)</ref>. | In the middle of the cleft located a <scene name='69/691577/Dxd_mn2/1'>DXD motif</scene>, highly conserved in a large number of glycosyltransferases, which coordinates the Mn2+ ion and was suggested to have a role in catalysis<ref>Busch, C. et al. J. Biol. Chem. 273, 19566−19572 (1998)</ref>. | ||
| Line 38: | Line 38: | ||
'''Arg/Gly 176''' | '''Arg/Gly 176''' | ||
| - | + | There are three conformations for the GTs enzymes, "open", "semi closed" and "closed" as they go from unliganded to the liganded states. | |
| + | the open conformation for the enzyme, in the absence of donor or acceptor, the nine C-terminal residues are disordered and a major portion of the internal loop (amino acid residues 176-195) is disordered, the semi closed and closed forms of the enzymes are generated by binding of UDP or of UDP and H antigen, respectively. The closed form is distinguished from the semiclosed form by the ordering of the final nine C-terminal residues through the formation of hydrogen bonds to both UDP and H antigen. The semi-closed forms for various mutants generally show significantly more disorder than the open forms, whereas the closed forms display little or no disorder depending strongly on the identity of residue 176. | ||
| + | |||
| + | The side chain of the amino acid residue itself does not make any contact with any other part of the enzyme in any structure but greatly influences the level of flexibility in both the internal loop and the C-terminal region. | ||
'''Gly/Ser 235''' | '''Gly/Ser 235''' | ||
| Line 50: | Line 53: | ||
Gly/Ala 268 can contact only moieties of the donor sugar residues that are identical in UDP-GalNAc and UDP-Gal (specifically hydroxyl groups O3 and O4), which does not explain how this residue could contribute to substrate specificity. | Gly/Ala 268 can contact only moieties of the donor sugar residues that are identical in UDP-GalNAc and UDP-Gal (specifically hydroxyl groups O3 and O4), which does not explain how this residue could contribute to substrate specificity. | ||
| + | |||
Therefore, the ability of the two enzymes to distinguish between the A and B donors is largely determined by '''a single amino acid residue'''. | Therefore, the ability of the two enzymes to distinguish between the A and B donors is largely determined by '''a single amino acid residue'''. | ||
Current revision
| |||||||||||
References
- ↑ Patenaude SI, Seto NO, Borisova SN, Szpacenko A, Marcus SL, Palcic MM, Evans SV. The structural basis for specificity in human ABO(H) blood group biosynthesis. Nat Struct Biol. 2002 Sep;9(9):685-90. PMID:12198488 doi:http://dx.doi.org/10.1038/nsb832
- ↑ Busch, C. et al. J. Biol. Chem. 273, 19566−19572 (1998)