Chemical communication in arthropods
From Proteopedia
| (6 intermediate revisions not shown.) | |||
| Line 8: | Line 8: | ||
In both [http://en.wikipedia.org/wiki/Arthropod arthropods] and [http://en.wikipedia.org/wiki/Vertebrate vertebrates] the detection of volatiles is completed by a complicated process which is mediated by soluble as well as transmembrane proteins <ref name="pelosi">DOI: 10.3389/fphys.2014.00320</ref>. | In both [http://en.wikipedia.org/wiki/Arthropod arthropods] and [http://en.wikipedia.org/wiki/Vertebrate vertebrates] the detection of volatiles is completed by a complicated process which is mediated by soluble as well as transmembrane proteins <ref name="pelosi">DOI: 10.3389/fphys.2014.00320</ref>. | ||
It should be mentioned that the detection of [http://en.wikipedia.org/wiki/Pheromone pheromones] is also vital to microorganisms, as it regulates gene expression in what is termed [http://en.wikipedia.org/wiki/Quorum_sensing “quorum sensing”]. | It should be mentioned that the detection of [http://en.wikipedia.org/wiki/Pheromone pheromones] is also vital to microorganisms, as it regulates gene expression in what is termed [http://en.wikipedia.org/wiki/Quorum_sensing “quorum sensing”]. | ||
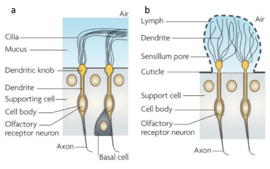
| - | In arthropods, most of what is known on chemosensory communication is based on | + | In arthropods, most of what is known on chemosensory communication is based on research studies in insects. The process begins when a volatile (mostly a small [http://en.wikipedia.org/wiki/Hydrophobe hydrophobic] molecule) enters the chemosensilla lymph of an insect, or the mucus of a vertebrate in the nasal cavity ([[fig 1]]). Both media are abundant in soluble proteins which bind to the hydrophobic molecules, solubilize and carry the molecule to the [http://en.wikipedia.org/wiki/Chemoreceptor chemoreceptors] on the dendritic membrane of the olfactory receptor neuron <ref name="kaupp" /><ref name="vogt">Vogt RG (2005) Molecular basis of pheromone detection in insects. Comprehensive Insect Physiology, Biochemistry, Pharmacology and Molecular Biology, eds Gilbert LI, Iatro K, Gills S (Elsevier, London), Vol 3, pp 753–804.</ref>.The chemical signal is thereby translated into an electrical signal which can cause an immediate response, or further processed with other signals in the insect's mushroom bodies or vertebrate's brain ([[fig 2]])<ref>doi: 10.3389/fncel.2012.00048</ref><ref>doi: 10.1146/annurev-ento-120811-153635</ref>. |
| - | == What | + | == What are the differences and similarities between Arthropods and Vertebrates? == |
Though functionally similar, receptors as well as soluble proteins are structurally and genetically unrelated in insects and vertebrates (see [[fig 3]] for the putative evolution of proteins involved in chemosensory system). | Though functionally similar, receptors as well as soluble proteins are structurally and genetically unrelated in insects and vertebrates (see [[fig 3]] for the putative evolution of proteins involved in chemosensory system). | ||
*'''Receptors''' | *'''Receptors''' | ||
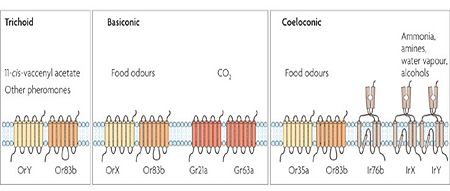
| - | Most of the vertebrates' chemosensory receptors are metabotropic and belong to the [[G protein-coupled receptors]]. Once the volatile binds to the receptor it initiates intracellular signal transduction <ref>doi: 10.1016/S0167-4838(00)00167-9</ref>. On the other hand, arthropods' and insects' chemoreceptors are composed of two subunits: [http://en.wikipedia.org/wiki/Olfactory_receptor Receptor] and [http://en.wikipedia.org/wiki/Co-receptor Co-receptor] that upon interaction with the volatile or the complex of volatile-soluble protein, are activated and serve as an [http://en.wikipedia.org/wiki/Ion_channel ion channel]. The opening of the ion channel changes the [http://en.wikipedia.org/wiki/Membrane_potential membrane potential], and starts the inter-cellular signal transduction<ref | + | Most of the vertebrates' chemosensory receptors are metabotropic and belong to the [[G protein-coupled receptors]]. Once the volatile binds to the receptor it initiates intracellular signal transduction <ref>doi: 10.1016/S0167-4838(00)00167-9</ref>. On the other hand, arthropods' and insects' chemoreceptors are composed of two subunits: [http://en.wikipedia.org/wiki/Olfactory_receptor Receptor] and [http://en.wikipedia.org/wiki/Co-receptor Co-receptor] that upon interaction with the volatile or the complex of volatile-soluble protein, are activated and serve as an [http://en.wikipedia.org/wiki/Ion_channel ion channel]<ref>doi: 10.1038/nature06861</ref>. The opening of the ion channel changes the [http://en.wikipedia.org/wiki/Membrane_potential membrane potential], and starts the inter-cellular signal transduction<ref name="vogt" />.[[Image:Chemoreceptors-insects.jpg|thumb|center|upright=2.5|Figure 4: Types of insect receptors. Figure 1 from Kaupp (2010), used with permission of Prof. U. Benjamin Kaupp.]] |
*'''Soluble proteins''' | *'''Soluble proteins''' | ||
These proteins which are concentrated in the sensillar lymph, solubilize and carry the volatile molecules to the receptor. | These proteins which are concentrated in the sensillar lymph, solubilize and carry the volatile molecules to the receptor. | ||
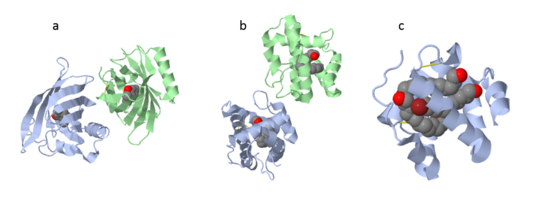
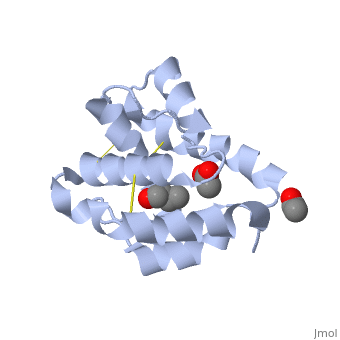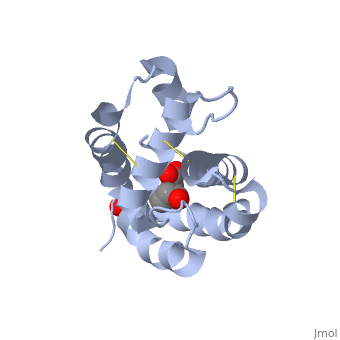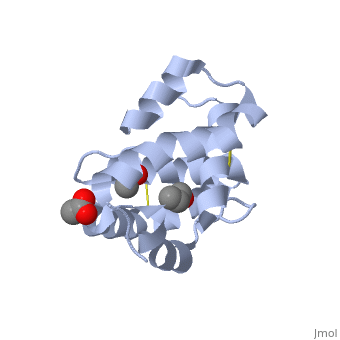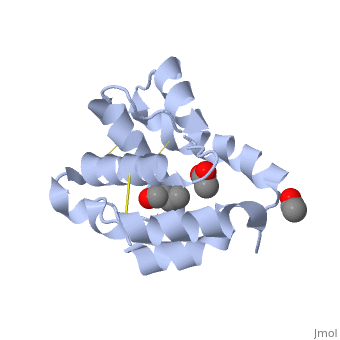
| - | There are two main known types of soluble proteins that are involved in arthropods' chemical communication: '''[http://proteopedia.org/w/Odorant_binding_protein Odorant binding proteins –OBPs]''','''[http://proteopedia.org/wiki/index.php/Chemosensory_protein Chemosensory protein-CSP]''' ([[fig 5]]). Though bearing the same name and participating in the same function, OBP of vertebrates and arthropods are two distinct families with completely different structure and origin<ref name="pelosi" />. Arthropods' OBP are composed of alpha helices, while vertebrates' OBP belong to the [http://en.wikipedia.org/wiki/Lipocalin Lipocalins] super family and have a beta-barrel structure (for structure comparison, see [[table 1]] and [[fig 5]]). Recently, another family of | + | There are two main known types of soluble proteins that are involved in arthropods' chemical communication: '''[http://proteopedia.org/w/Odorant_binding_protein Odorant binding proteins –OBPs]''','''[http://proteopedia.org/wiki/index.php/Chemosensory_protein Chemosensory protein-CSP]''' ([[fig 5]]). Though bearing the same name and participating in the same function, OBP of vertebrates and arthropods are two distinct families with completely different structure and origin<ref name="pelosi" />. Arthropods' OBP are composed of alpha helices, while vertebrates' OBP belong to the [http://en.wikipedia.org/wiki/Lipocalin Lipocalins] super family and have a beta-barrel structure (for structure comparison, see [[table 1]] and [[fig 5]]). Recently, another family of proteins has been suggested to play a role in ant chemical communication, '''[https://www.wikigenes.org/e/gene/e/10577.html Niemann-Pick type C2 protein-NPC2]''' <ref name="ishida">DOI: 10.1073/pnas.1323928111</ref>. |
[[Image:Fig 3 soluble proteins.png|thumb|center|upright=3|Figure 5. (a) An example for vertebrate's OBP-a pig OBP, PDB:[[1e06]]; (b) An example for insect's OBP- ''Bombyx mori'' PBP, PDB:[[1dqe]]; (c) An example for insect's CSP-''Mamestra brassicae'' CSP2 PDB:[[1n8u]]]] | [[Image:Fig 3 soluble proteins.png|thumb|center|upright=3|Figure 5. (a) An example for vertebrate's OBP-a pig OBP, PDB:[[1e06]]; (b) An example for insect's OBP- ''Bombyx mori'' PBP, PDB:[[1dqe]]; (c) An example for insect's CSP-''Mamestra brassicae'' CSP2 PDB:[[1n8u]]]] | ||
[[Image:Soluble proteins table.png|thumb|center|upright=2|Table 1. Summation of the main structure properties of soluble proteins types]] | [[Image:Soluble proteins table.png|thumb|center|upright=2|Table 1. Summation of the main structure properties of soluble proteins types]] | ||
| Line 26: | Line 26: | ||
==Types of Soluble proteins in arthropods== | ==Types of Soluble proteins in arthropods== | ||
<StructureSection load='1OOH' size='340' side='right' caption='''Mamestra brassicae'' CSP2 PDB:[[1n8u]]' scene=''> | <StructureSection load='1OOH' size='340' side='right' caption='''Mamestra brassicae'' CSP2 PDB:[[1n8u]]' scene=''> | ||
| - | In each protein the | + | In each protein the conserved <font color=#FF7E00><b>'''cysteins'''</b></font>and the <font color=#FDEE00><b>'''disulfide bonds'''</b></font> are color marked. |
*'''OBP''' | *'''OBP''' | ||
| - | This family was the first soluble protein discovered in the chemosensory system of arthropods. Its general strcuture is of alpha helices that are compactly tied by 3 | + | This family was the first soluble protein discovered in the chemosensory system of arthropods. Its general strcuture is of alpha helices that are compactly tied by 3 disulfide bridges formed by 6 conserved cystein residues. |
| - | The male fly of ''Drosophila melanogaster'' | + | The male fly of ''Drosophila melanogaster'' produces the pheromone 11-cis vaccenyl acetate which mediates aggregation behavior of other flies of the same species<ref>doi:10.1523/JNEUROSCI.0876-06.2006</ref>. The detection of the pheromone, was shown to be mediated by pheromone-induced conformational shifts in the PBP, <scene name='61/614066/Lush/1'>LUSH</scene>. In fact, the triggering of the neuron was possible in the absence of the pheromone itself<ref>doi: m10.1016/j.cell.2008.04.046</ref>. |
*'''CSP''' | *'''CSP''' | ||
| Line 37: | Line 37: | ||
*'''NPC2''' | *'''NPC2''' | ||
| - | Recently, a new family of proteins have been suggested to play a role as a soluble protein. Until now NPC2 proteins were known to carry lipids and cholesterol molecules in the cells<ref>doi: 10.1016/j.bbalip.2004.08.007</ref>, yet a member of this family was isolated from the antennae of the ant ''Camponatus japonicus''<ref name="ishida" / | + | Recently, a new family of proteins have been suggested to play a role as a soluble protein. Until now NPC2 proteins were known to carry lipids and cholesterol molecules in the cells<ref>doi: 10.1016/j.bbalip.2004.08.007</ref>, yet a member of this family was isolated from the antennae of the ant ''Camponatus japonicus''<ref name="ishida" />. This protein has a beta-barrel shape and <scene name='61/614066/Npc2_cysteins/2'>6 cysteins forming 3 disulfide bonds</scene> (similar to classical vertebrates OBP). Another conserved feature is the<scene name='61/614066/Resid_in_the_cavity_gate/2'>6 conserved hydrophobic residues at the gate of the cavity</scene>, supposedly attracting the hydrophobic ligand to the cavity<ref>doi: 10.1074/jbc.M703848200.STRUCTURAL</ref>. This first finding could explain the small number of known soluble proteins in some insects and other arthropods, relativity to their ability to sense large number of volatiles. |
| - | + | ||
| + | </StructureSection> | ||
== See also == | == See also == | ||
*[[Odorant_binding_protein_3D_structures]] | *[[Odorant_binding_protein_3D_structures]] | ||
Current revision

Contents |
The molecular basis of chemical communication
The sense of smell, Olfaction is a primary sense in nature. It plays a significant role in behaviors which are crucial for the organism survival: food searching, host and mating selection, and avoiding predators and pathogens [3].
In both arthropods and vertebrates the detection of volatiles is completed by a complicated process which is mediated by soluble as well as transmembrane proteins [4]. It should be mentioned that the detection of pheromones is also vital to microorganisms, as it regulates gene expression in what is termed “quorum sensing”. In arthropods, most of what is known on chemosensory communication is based on research studies in insects. The process begins when a volatile (mostly a small hydrophobic molecule) enters the chemosensilla lymph of an insect, or the mucus of a vertebrate in the nasal cavity (fig 1). Both media are abundant in soluble proteins which bind to the hydrophobic molecules, solubilize and carry the molecule to the chemoreceptors on the dendritic membrane of the olfactory receptor neuron [3][5].The chemical signal is thereby translated into an electrical signal which can cause an immediate response, or further processed with other signals in the insect's mushroom bodies or vertebrate's brain (fig 2)[6][7].
What are the differences and similarities between Arthropods and Vertebrates?
Though functionally similar, receptors as well as soluble proteins are structurally and genetically unrelated in insects and vertebrates (see fig 3 for the putative evolution of proteins involved in chemosensory system).
- Receptors
- Soluble proteins
These proteins which are concentrated in the sensillar lymph, solubilize and carry the volatile molecules to the receptor. There are two main known types of soluble proteins that are involved in arthropods' chemical communication: Odorant binding proteins –OBPs,Chemosensory protein-CSP (fig 5). Though bearing the same name and participating in the same function, OBP of vertebrates and arthropods are two distinct families with completely different structure and origin[4]. Arthropods' OBP are composed of alpha helices, while vertebrates' OBP belong to the Lipocalins super family and have a beta-barrel structure (for structure comparison, see table 1 and fig 5). Recently, another family of proteins has been suggested to play a role in ant chemical communication, Niemann-Pick type C2 protein-NPC2 [10].
Types of Soluble proteins in arthropods
| |||||||||||
See also
- Odorant_binding_protein_3D_structures
- For comprehensive explanation about quorum sensing please turn to Fuqua et al. (2001) [16].
- for more information about the protein-ligand interaction, you may go to Odorant binding protein.
References
- ↑ Sanchez-Gracia A, Vieira FG, Rozas J. Molecular evolution of the major chemosensory gene families in insects. Heredity (Edinb). 2009 Sep;103(3):208-16. doi: 10.1038/hdy.2009.55. Epub 2009 May, 13. PMID:19436326 doi:http://dx.doi.org/10.1038/hdy.2009.55
- ↑ Vieira FG, Rozas J. Comparative genomics of the odorant-binding and chemosensory protein gene families across the Arthropoda: origin and evolutionary history of the chemosensory system. Genome Biol Evol. 2011;3:476-90. doi: 10.1093/gbe/evr033. Epub 2011 Apr 28. PMID:21527792 doi:http://dx.doi.org/10.1093/gbe/evr033
- ↑ 3.0 3.1 Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci. 2010 Mar;11(3):188-200. doi: 10.1038/nrn2789. Epub 2010 Feb 10. PMID:20145624 doi:http://dx.doi.org/10.1038/nrn2789
- ↑ 4.0 4.1 Pelosi P, Iovinella I, Felicioli A, Dani FR. Soluble proteins of chemical communication: an overview across arthropods. Front Physiol. 2014 Aug 27;5:320. doi: 10.3389/fphys.2014.00320. eCollection, 2014. PMID:25221516 doi:http://dx.doi.org/10.3389/fphys.2014.00320
- ↑ 5.0 5.1 Vogt RG (2005) Molecular basis of pheromone detection in insects. Comprehensive Insect Physiology, Biochemistry, Pharmacology and Molecular Biology, eds Gilbert LI, Iatro K, Gills S (Elsevier, London), Vol 3, pp 753–804.
- ↑ Wicher D. Functional and evolutionary aspects of chemoreceptors. Front Cell Neurosci. 2012 Oct 26;6:48. doi: 10.3389/fncel.2012.00048. eCollection, 2012. PMID:23112762 doi:http://dx.doi.org/10.3389/fncel.2012.00048
- ↑ Leal WS. Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu Rev Entomol. 2013;58:373-91. doi: 10.1146/annurev-ento-120811-153635. Epub, 2012 Sep 27. PMID:23020622 doi:http://dx.doi.org/10.1146/annurev-ento-120811-153635
- ↑ doi: https://dx.doi.org/10.1016/S0167-4838(00)00167-9
- ↑ Wicher D, Schafer R, Bauernfeind R, Stensmyr MC, Heller R, Heinemann SH, Hansson BS. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008 Apr 24;452(7190):1007-11. doi: 10.1038/nature06861. Epub 2008 Apr, 13. PMID:18408711 doi:http://dx.doi.org/10.1038/nature06861
- ↑ 10.0 10.1 Ishida Y, Tsuchiya W, Fujii T, Fujimoto Z, Miyazawa M, Ishibashi J, Matsuyama S, Ishikawa Y, Yamazaki T. Niemann-Pick type C2 protein mediating chemical communication in the worker ant. Proc Natl Acad Sci U S A. 2014 Mar 11;111(10):3847-52. doi:, 10.1073/pnas.1323928111. Epub 2014 Feb 24. PMID:24567405 doi:http://dx.doi.org/10.1073/pnas.1323928111
- ↑ Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006 Aug 23;26(34):8727-33. PMID:16928861 doi:10.1523/JNEUROSCI.0876-06.2006
- ↑ doi: https://dx.doi.org/m10.1016/j.cell.2008.04.046
- ↑ Campanacci V, Lartigue A, Hallberg BM, Jones TA, Giudici-Orticoni MT, Tegoni M, Cambillau C. Moth chemosensory protein exhibits drastic conformational changes and cooperativity on ligand binding. Proc Natl Acad Sci U S A. 2003 Apr 29;100(9):5069-74. Epub 2003 Apr 15. PMID:12697900 doi:10.1073/pnas.0836654100
- ↑ Vanier MT, Millat G. Structure and function of the NPC2 protein. Biochim Biophys Acta. 2004 Oct 11;1685(1-3):14-21. PMID:15465422 doi:http://dx.doi.org/10.1016/j.bbalip.2004.08.007
- ↑ doi: https://dx.doi.org/10.1074/jbc.M703848200.STRUCTURAL
- ↑ Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439-68. PMID:11700290 doi:http://dx.doi.org/10.1146/annurev.genet.35.102401.090913