We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
UBC13 MMS2
From Proteopedia
(Difference between revisions)
| (107 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <Structure load='1J7D' size='350' scene='69/695700/Ubc13_mms2_overall/1' caption='Human ubiquitin conjugating enzyme E2 13 complex with HMM2 (PDB code [[1j7d]])' > | |
| - | < | + | |
| - | + | ==Summary== | |
| + | |||
| + | '''Ubc13''' is an '''E2 ubiquitin-conjugating enzyme''' that can form a heterodimer with Mms2 to function as a part of the translesion synthesis (TLS) pathway,<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref><ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. When bound to Mms2, Ubc13 will polyubiquitinate proliferating cell nuclear antigen (PCNA), a sliding clamp protein at the DNA transcription fork<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref>. Ubc13-Mms2 functions to polyubiquitinate PCNA following the initial monoubiquitination by Rad6-Rad18 (another E2 complex)<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. It is important to note that Ubc13 lacks the ability to be catalytically active without Mms2, hinting at inaccuracies within the statement "structure determines function." | ||
== Function == | == Function == | ||
| - | == | + | Ubc13 functions as a heterodimer with Mms2, a structurally similar protein to Ubc13 that lacks the catalytic cysteine residue in the active site<ref name=mckenna>5. McKenna, S.; Spyracopoulos, L. F.; Moraes, T. F.; Pastushok, L. F.; Ptak, C. F.; Xiao W FAU - Ellison,,M.J.; Ellison, M. J. Noncovalent interaction between ubiquitin and the human DNA repair protein Mms2 is required for Ubc13-mediated polyubiquitination. The Journal of biological chemistry JID - 2985121R 1207.</ref><ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. The Ubc13-E2 complex with Mms2 functions primarily to enhance DNA repair from double stranded breaks. Mms2 bound to Ubc13 helps orient the ubiquitin molecule for proper ubiquitination of the K63 residue<ref name=vandemark>9. VanDemark, A. P.; FAU, H. R.; Tsui C FAU - Pickart,,C.M.; FAU, P. C.; Wolberger, C. Molecular insights into polyubiquitin chain assembly: crystal structure of the Mms2/Ubc13 heterodimer. Cell JID - 0413066 0726.</ref>. Mms2 is considered a Ubiquitin E2 variant (UEV) protein, because it lacks the catalytic cysteine residue necessary for proper thioester formation<ref name=vandemark>9. VanDemark, A. P.; FAU, H. R.; Tsui C FAU - Pickart,,C.M.; FAU, P. C.; Wolberger, C. Molecular insights into polyubiquitin chain assembly: crystal structure of the Mms2/Ubc13 heterodimer. Cell JID - 0413066 0726.</ref><ref name=moraes>6. Moraes, T. F.; FAU, E. R.; McKenna, S. F.; Pastushok, L. F.; Xiao W FAU - Glover,,J.N.; FAU, G. J.; Ellison, M. J. Crystal structure of the human ubiquitin conjugating enzyme complex, hMms2-hUbc13. Nature structural biology JID - 9421566 0816.</ref><ref name=mckenna>5. McKenna, S.; Spyracopoulos, L. F.; Moraes, T. F.; Pastushok, L. F.; Ptak, C. F.; Xiao W FAU - Ellison,,M.J.; Ellison, M. J. Noncovalent interaction between ubiquitin and the human DNA repair protein Mms2 is required for Ubc13-mediated polyubiquitination. The Journal of biological chemistry JID - 2985121R 1207.</ref>. |
| - | == | + | == Regulation == |
| - | == | + | The regulation of Ubc13 is controlled by the competitive binding of the two different UEV's, Mms2 and UEV1A<ref name=anderson> Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. Ubc13 binding to Mms2 activates the DNA repair pathway, while Ubc13 binding to UEV1A activates the NF-kappaB pathway, a gene regulation pathway involved in DNA transcription factors<ref name=anderson> Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. These two opposing pathways being downstream targets for Ubc13-bound complexes is the basis for regulation. |
| - | + | ==Coordinating Enzymes== | |
| - | </ | + | * Another E2 complex, Rad6-Rad18, starts the process of DNA repair by monoubiquitinating PCNA near the replication fork of DNA. This DNA repair will arrest cell cycle progression until DNA repair is complete<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. |
| + | * UEV1A, another cofactor enzyme that binds to Ubc13, is thought to compete with Mms2 for binding to Ubc13. This is thought to be a regulatory mechanism for Ubc13 activity in the nucleus of cells<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. | ||
| + | *Several DNA polymerases such as rev1, pol eta, and pol zeta contain Ubiquitin-binding domains that recognize <scene name='69/695700/Pcna/1'>K164</scene> polyubiquitination of PCNA<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref>. | ||
| + | *Rad5, an E3 RING (Really Interesting New Gene) protein, interacts with the Ubc13-Mms2 heterodimer in order to ligate the ubiquitin on the PCNA. Rad5, as well as Rad18 (RING proteins) are involved in the recruitment of Ubc13-Mms2 heterodimer formation<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. | ||
| + | |||
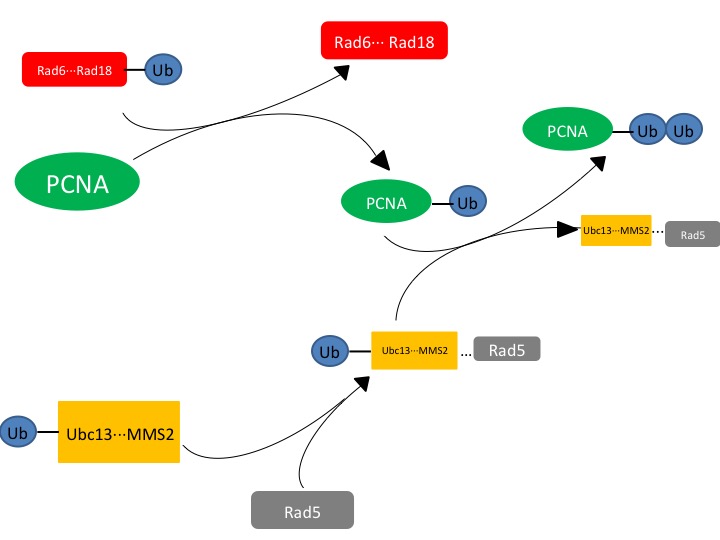
| + | == Pathway for DNA Repair == | ||
| + | [[Image:Slide2.jpg]] | ||
| + | |||
| + | |||
| + | '''Figure. 1. The affected protein PCNA is a DNA clamp that is monoubiquitinated by the Rad6-Rad18 complex. The monoubiquitinated PCNA is then polyubuiquitinated by the UBC13-Mms2-Rad5 complex.''' | ||
| + | |||
| + | |||
| + | == Structural Highlights/Important Residues == | ||
| + | |||
| + | Ubc13 weights 17.6 kDA, and Mms2 is 16.8 kDA<ref name=Pastushok> Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. The heterodimer is stable at high stalt concentrations (1 M), suggesting strong interactions between the two. Kd between the Ubc13 and Mms2 is 2 uM. | ||
| + | <scene name='69/695700/Ubc13_mms2_phe57/1'>Phe57</scene> and <scene name='69/695700/Ubc13_mms2_glu55/1'>Glu55</scene> of Ubc13 interact with the N-terminal domain of Mms2 to ensure stable docking<ref name=Pastushok> Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. Additionally, <scene name='69/695700/Ubc13_mms2_arg70/1'>Arg70</scene> hydrophobically interacts with an alpha helix of Mms2 in two places<ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. Mms2’s <scene name='69/695700/Ubc13_mms2_phe13/1'>Phe13</scene> is inserted between Glu55, Phe57, and Arg70 of Ubc13 to create a <scene name='69/695700/Ubc13_mms2_hydrophobic/1'>hydrophobic pocket</scene><ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. It is therorized that <scene name='69/695700/Ubc13_mms2_glu55_and_arg70/1'>Glu55 and Arg70</scene> of Ubc13 are more important for recognition instead of stability<ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. | ||
| + | |||
| + | ==Mechanism== | ||
| + | |||
| + | The exact mechanism for how Ubc13 transfers ubiquitin is not known, however the mechanism occurs in either a step-wise or concerted reaction. Ubc13, as an E2, froms a covalent bond with ubiquitin and then transfers the ubiquitin to the target protein via a thioester intermediate. Ubiquitin is removed from Ubc13 and Mms2 complex and placed onto PCNA. | ||
| + | |||
| + | ==3D structure of Ubc13== | ||
| + | |||
| + | [[Ubiquitin conjugating enzyme]] | ||
== References == | == References == | ||
| + | |||
<references/> | <references/> | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
David A Taves, Michal Harel, Christopher Alexander Hudson, Nicholas R. Dunham