We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
UBC13 MMS2
From Proteopedia
(Difference between revisions)
| (18 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <Structure load='1J7D' size='350 | + | <Structure load='1J7D' size='350' scene='69/695700/Ubc13_mms2_overall/1' caption='Human ubiquitin conjugating enzyme E2 13 complex with HMM2 (PDB code [[1j7d]])' > |
==Summary== | ==Summary== | ||
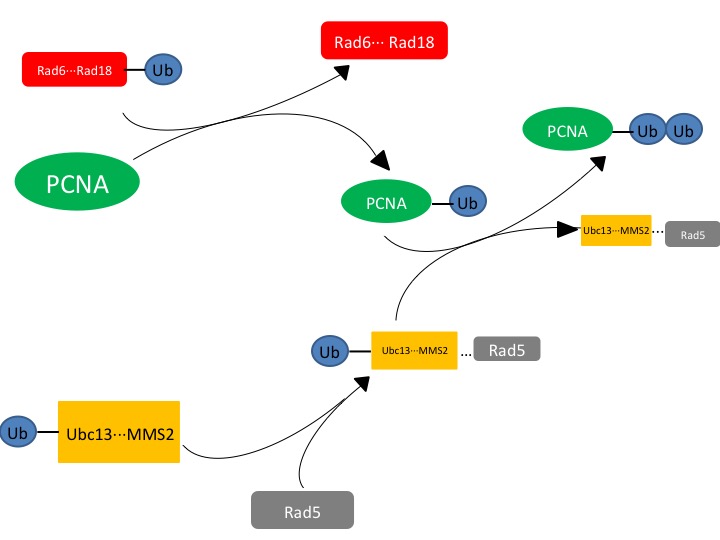
| - | Ubc13 is an E2 ubiquitin-conjugating enzyme that can form a heterodimer with Mms2 to function as a part of the translesion synthesis (TLS) pathway,<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref><ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. When bound to Mms2, Ubc13 will polyubiquitinate proliferating cell nuclear antigen (PCNA), a sliding clamp protein at the DNA transcription fork<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref>. Ubc13-Mms2 functions to polyubiquitinate PCNA following the initial monoubiquitination by Rad6-Rad18 (another E2 complex)<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. | + | '''Ubc13''' is an '''E2 ubiquitin-conjugating enzyme''' that can form a heterodimer with Mms2 to function as a part of the translesion synthesis (TLS) pathway,<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref><ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. When bound to Mms2, Ubc13 will polyubiquitinate proliferating cell nuclear antigen (PCNA), a sliding clamp protein at the DNA transcription fork<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref>. Ubc13-Mms2 functions to polyubiquitinate PCNA following the initial monoubiquitination by Rad6-Rad18 (another E2 complex)<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. It is important to note that Ubc13 lacks the ability to be catalytically active without Mms2, hinting at inaccuracies within the statement "structure determines function." |
== Function == | == Function == | ||
| - | Ubc13 functions as a heterodimer with Mms2, a structurally similar protein to Ubc13 that lacks the catalytic cysteine residue in the active site<ref name=mckenna>5. McKenna, S.; Spyracopoulos, L. F.; Moraes, T. F.; Pastushok, L. F.; Ptak, C. F.; Xiao W FAU - Ellison,,M.J.; Ellison, M. J. Noncovalent interaction between ubiquitin and the human DNA repair protein Mms2 is required for Ubc13-mediated polyubiquitination. The Journal of biological chemistry JID - 2985121R 1207.</ref><ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. The Ubc13-E2 complex with Mms2 functions primarily to enhance DNA repair from double stranded breaks. Mms2 bound to Ubc13 helps orient the ubiquitin molecule for proper ubiquitination of the K63 residue. Mms2 is considered a Ubiquitin E2 variant (UEV) protein, because it lacks the catalytic cysteine residue necessary for proper thioester formation<ref name=moraes>6. Moraes, T. F.; FAU, E. R.; McKenna, S. F.; Pastushok, L. F.; Xiao W FAU - Glover,,J.N.; FAU, G. J.; Ellison, M. J. Crystal structure of the human ubiquitin conjugating enzyme complex, hMms2-hUbc13. Nature structural biology JID - 9421566 0816.</ref><ref name=mckenna>5. McKenna, S.; Spyracopoulos, L. F.; Moraes, T. F.; Pastushok, L. F.; Ptak, C. F.; Xiao W FAU - Ellison,,M.J.; Ellison, M. J. Noncovalent interaction between ubiquitin and the human DNA repair protein Mms2 is required for Ubc13-mediated polyubiquitination. The Journal of biological chemistry JID - 2985121R 1207.</ref>. | + | Ubc13 functions as a heterodimer with Mms2, a structurally similar protein to Ubc13 that lacks the catalytic cysteine residue in the active site<ref name=mckenna>5. McKenna, S.; Spyracopoulos, L. F.; Moraes, T. F.; Pastushok, L. F.; Ptak, C. F.; Xiao W FAU - Ellison,,M.J.; Ellison, M. J. Noncovalent interaction between ubiquitin and the human DNA repair protein Mms2 is required for Ubc13-mediated polyubiquitination. The Journal of biological chemistry JID - 2985121R 1207.</ref><ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. The Ubc13-E2 complex with Mms2 functions primarily to enhance DNA repair from double stranded breaks. Mms2 bound to Ubc13 helps orient the ubiquitin molecule for proper ubiquitination of the K63 residue<ref name=vandemark>9. VanDemark, A. P.; FAU, H. R.; Tsui C FAU - Pickart,,C.M.; FAU, P. C.; Wolberger, C. Molecular insights into polyubiquitin chain assembly: crystal structure of the Mms2/Ubc13 heterodimer. Cell JID - 0413066 0726.</ref>. Mms2 is considered a Ubiquitin E2 variant (UEV) protein, because it lacks the catalytic cysteine residue necessary for proper thioester formation<ref name=vandemark>9. VanDemark, A. P.; FAU, H. R.; Tsui C FAU - Pickart,,C.M.; FAU, P. C.; Wolberger, C. Molecular insights into polyubiquitin chain assembly: crystal structure of the Mms2/Ubc13 heterodimer. Cell JID - 0413066 0726.</ref><ref name=moraes>6. Moraes, T. F.; FAU, E. R.; McKenna, S. F.; Pastushok, L. F.; Xiao W FAU - Glover,,J.N.; FAU, G. J.; Ellison, M. J. Crystal structure of the human ubiquitin conjugating enzyme complex, hMms2-hUbc13. Nature structural biology JID - 9421566 0816.</ref><ref name=mckenna>5. McKenna, S.; Spyracopoulos, L. F.; Moraes, T. F.; Pastushok, L. F.; Ptak, C. F.; Xiao W FAU - Ellison,,M.J.; Ellison, M. J. Noncovalent interaction between ubiquitin and the human DNA repair protein Mms2 is required for Ubc13-mediated polyubiquitination. The Journal of biological chemistry JID - 2985121R 1207.</ref>. |
== Regulation == | == Regulation == | ||
| Line 17: | Line 17: | ||
* Another E2 complex, Rad6-Rad18, starts the process of DNA repair by monoubiquitinating PCNA near the replication fork of DNA. This DNA repair will arrest cell cycle progression until DNA repair is complete<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. | * Another E2 complex, Rad6-Rad18, starts the process of DNA repair by monoubiquitinating PCNA near the replication fork of DNA. This DNA repair will arrest cell cycle progression until DNA repair is complete<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. | ||
* UEV1A, another cofactor enzyme that binds to Ubc13, is thought to compete with Mms2 for binding to Ubc13. This is thought to be a regulatory mechanism for Ubc13 activity in the nucleus of cells<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. | * UEV1A, another cofactor enzyme that binds to Ubc13, is thought to compete with Mms2 for binding to Ubc13. This is thought to be a regulatory mechanism for Ubc13 activity in the nucleus of cells<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. | ||
| - | *Several DNA polymerases such as rev1, pol eta, and pol zeta contain Ubiquitin-binding domains that recognize K164 polyubiquitination of PCNA<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref>. | + | *Several DNA polymerases such as rev1, pol eta, and pol zeta contain Ubiquitin-binding domains that recognize <scene name='69/695700/Pcna/1'>K164</scene> polyubiquitination of PCNA<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref>. |
*Rad5, an E3 RING (Really Interesting New Gene) protein, interacts with the Ubc13-Mms2 heterodimer in order to ligate the ubiquitin on the PCNA. Rad5, as well as Rad18 (RING proteins) are involved in the recruitment of Ubc13-Mms2 heterodimer formation<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. | *Rad5, an E3 RING (Really Interesting New Gene) protein, interacts with the Ubc13-Mms2 heterodimer in order to ligate the ubiquitin on the PCNA. Rad5, as well as Rad18 (RING proteins) are involved in the recruitment of Ubc13-Mms2 heterodimer formation<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. | ||
| Line 30: | Line 30: | ||
Ubc13 weights 17.6 kDA, and Mms2 is 16.8 kDA<ref name=Pastushok> Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. The heterodimer is stable at high stalt concentrations (1 M), suggesting strong interactions between the two. Kd between the Ubc13 and Mms2 is 2 uM. | Ubc13 weights 17.6 kDA, and Mms2 is 16.8 kDA<ref name=Pastushok> Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. The heterodimer is stable at high stalt concentrations (1 M), suggesting strong interactions between the two. Kd between the Ubc13 and Mms2 is 2 uM. | ||
| - | Phe57 and Glu55 of Ubc13 interact with the N-terminal domain of Mms2 to ensure stable docking<ref name=Pastushok> Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. Additionally, Arg70 hydrophobically interacts with an alpha helix of Mms2 in two places<ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. Mms2’s Phe13 is inserted between Glu55, Phe57, and Arg70 of Ubc13 to create a hydrophobic pocket<ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. It is therorized that Glu55 and Arg70 of Ubc13 are more important for recognition instead of stability<ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. | + | <scene name='69/695700/Ubc13_mms2_phe57/1'>Phe57</scene> and <scene name='69/695700/Ubc13_mms2_glu55/1'>Glu55</scene> of Ubc13 interact with the N-terminal domain of Mms2 to ensure stable docking<ref name=Pastushok> Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. Additionally, <scene name='69/695700/Ubc13_mms2_arg70/1'>Arg70</scene> hydrophobically interacts with an alpha helix of Mms2 in two places<ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. Mms2’s <scene name='69/695700/Ubc13_mms2_phe13/1'>Phe13</scene> is inserted between Glu55, Phe57, and Arg70 of Ubc13 to create a <scene name='69/695700/Ubc13_mms2_hydrophobic/1'>hydrophobic pocket</scene><ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. It is therorized that <scene name='69/695700/Ubc13_mms2_glu55_and_arg70/1'>Glu55 and Arg70</scene> of Ubc13 are more important for recognition instead of stability<ref name=Pastushok> 7. Pastushok, L.; FAU, M. T.; FAU, E. M.; Xiao, W. A single Mms2 "key" residue insertion into a Ubc13 pocket determines the interface specificity of a human Lys63 ubiquitin conjugation complex. The Journal of biological chemistry JID - 2985121R 0902.</ref>. |
==Mechanism== | ==Mechanism== | ||
| - | The exact mechanism for how Ubc13 transfers ubiquitin is not known, however the mechanism occurs in either a step-wise or concerted reaction. | + | The exact mechanism for how Ubc13 transfers ubiquitin is not known, however the mechanism occurs in either a step-wise or concerted reaction. Ubc13, as an E2, froms a covalent bond with ubiquitin and then transfers the ubiquitin to the target protein via a thioester intermediate. Ubiquitin is removed from Ubc13 and Mms2 complex and placed onto PCNA. |
| + | ==3D structure of Ubc13== | ||
| + | [[Ubiquitin conjugating enzyme]] | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
David A Taves, Michal Harel, Christopher Alexander Hudson, Nicholas R. Dunham