Ubc9
From Proteopedia
(Difference between revisions)
| (2 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <StructureSection load='1U9A' size='350' side='right' caption='Human Ubiquitin Conjugating Protein Ubc9 ' scene=''> | + | <StructureSection load='1U9A' size='350' side='right' caption='Human Ubiquitin Conjugating Protein Ubc9 (PDB code [[1u9a]])' scene=''> |
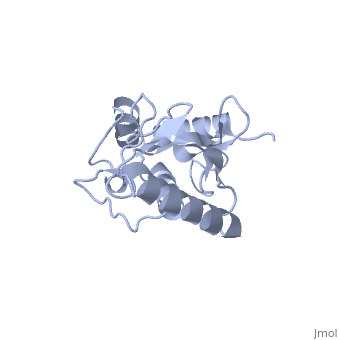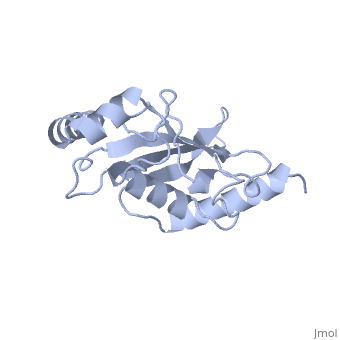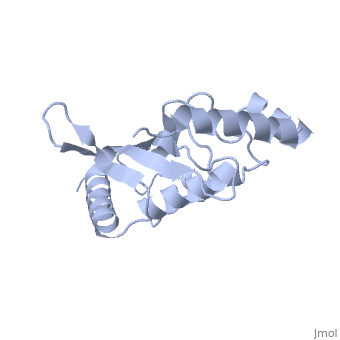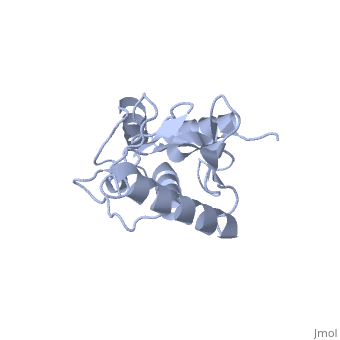
| - | ''Ubc9'' is a [[ubiquitin conjugating enzyme]] (E2) whose function involves the transfer of [[ubiquitin]] or [[small ubiquitin-like modifier]] (SUMO) from [[ubiquitin activating enzyme]] (E1) to its designated substrate. <scene name='69/694804/Ubc9_rainbow/1'>Ubc9</scene> is the only E2 enzyme specifically involved in SUMO transfer <ref name="crystal">PMID:9261152</ref><ref name="n terminal amino acids">PMID:25637535</ref>. | + | '''Ubc9''' is a [[ubiquitin conjugating enzyme]] (E2) whose function involves the transfer of [[ubiquitin]] or [[small ubiquitin-like modifier]] (SUMO) from [[ubiquitin activating enzyme]] (E1) to its designated substrate. <scene name='69/694804/Ubc9_rainbow/1'>Ubc9</scene> is the only E2 enzyme specifically involved in SUMO transfer <ref name="crystal">PMID:9261152</ref><ref name="n terminal amino acids">PMID:25637535</ref>. |
== Structure == | == Structure == | ||
| - | Murine/human | + | Murine/human Ubc9 exhibits a single domain structure consisting of both alpha helices and beta-pleated sheets (PDB: 1U9A). <scene name='69/694804/Cys93/6'>Cys93</scene> has been identified as the active residue and is located on a loop of amino acids (78-108) between beta sheet four and alpha helix two <ref name="crystal"/>. Amino acids surrounding the active site have been shown to play a role in catalysis of SUMO transfer to substrate via the suppression the the substrate lysine side chain pKa, which serves the purpose of nucleophile activation <ref name="lysine activation">PMID:16732283</ref>. |
A crystal structure has shown that Ubc9 is the central protein in the <font color='green'>SUMO</font>-<font color='F090A0'>RanGAP1</font>-<font color='blue'>Ubc9</font>-<font color='FFCC00'>Nup358</font> <scene name='69/694804/Rangap1-sumo-ubc9-nup538/1'>quaternary complex</scene> (PDB: 1Z5S), which forms in such a way as to enhance conjugation and transfer of the SUMO to substrate <ref name="quaternarycomplex">PMID:15931224</ref>. | A crystal structure has shown that Ubc9 is the central protein in the <font color='green'>SUMO</font>-<font color='F090A0'>RanGAP1</font>-<font color='blue'>Ubc9</font>-<font color='FFCC00'>Nup358</font> <scene name='69/694804/Rangap1-sumo-ubc9-nup538/1'>quaternary complex</scene> (PDB: 1Z5S), which forms in such a way as to enhance conjugation and transfer of the SUMO to substrate <ref name="quaternarycomplex">PMID:15931224</ref>. | ||
| Line 12: | Line 12: | ||
== Function == | == Function == | ||
| - | + | Ubc9 is enzymatically involved in the SUMOylation process, as it is responsible for ligating the SUMO to the protein. If the reaction occurs ''in vitro'', Ubc9 will ligate the SUMO directly to the substrate, while if the reaction occurs ''in vivo'', the SUMO will be ligated to the conjugating enzyme (E3) and then ligated onto the substrate <ref name="protein control">PMID:25097219</ref>. Initially in the SUMOylation pathway, a thioester bond is formed between the SUMO and the E1 enzyme via an ATP-dependent reaction. Next, the SUMO is transferred to the active cysteine of the E2, in this case, Ubc9. Finally, the SUMO is ligated to a lysine side chain amino group of the substrate, during which an E3 enzyme may or may not be recruited. The use of E3 mediated transfer serves functions such as increasing the specificity for the substrate to be SUMOylated <ref name="ubcsumocomplex"/>. | |
Ubc9 has been found to be predominantly nuclear, with its N-terminal amino acids being integral to its localization. Ubc9 nuclear accumulation is vital for normal SUMOylation rates <ref name="n terminal amino acids"/>. | Ubc9 has been found to be predominantly nuclear, with its N-terminal amino acids being integral to its localization. Ubc9 nuclear accumulation is vital for normal SUMOylation rates <ref name="n terminal amino acids"/>. | ||
| Line 20: | Line 20: | ||
SUMOylation has been shown to enhance processes such as DNA methyltransferse 1 enzymatic activity, which has a major regulatory effect on gene transcription <ref name="DNMT1 activity">PMID:19450230</ref>. The transcription factor AP-2 is SUMOylated by Ubc9, a process which decreases the transcription activation potential of AP-2, thus having an effect on gene transcription, and therefore likely having an effect on gene expression as well <ref name="factor AP2">PMID:12072432</ref>. | SUMOylation has been shown to enhance processes such as DNA methyltransferse 1 enzymatic activity, which has a major regulatory effect on gene transcription <ref name="DNMT1 activity">PMID:19450230</ref>. The transcription factor AP-2 is SUMOylated by Ubc9, a process which decreases the transcription activation potential of AP-2, thus having an effect on gene transcription, and therefore likely having an effect on gene expression as well <ref name="factor AP2">PMID:12072432</ref>. | ||
| - | Noncovalent interactions between Ubc9 and SUMO distant from the active site have been shown to be critical in the formation of SUMO chains, upon which ''in vivo'' functions such as metabolic regulation and signaling are dependent. Mutagenesis studies on the noncovalent | + | Noncovalent interactions between Ubc9 and SUMO distant from the active site have been shown to be critical in the formation of SUMO chains, upon which ''in vivo'' functions such as metabolic regulation and signaling are dependent. Mutagenesis studies on the noncovalent Ubc9-SUMO1 complex have shown that ''Ubc9H20D'' and ''SUMO1E67R'' mutations cause inhibition of noncovalent interaction between the two, and thus inhibit complex formation. A similar interaction is seen in the ''Ubc9''-SUMO2 complex <ref name="ubcsumocomplex"/>. Lack of noncovalent interaction between ''Ubc9'' and SUMO2 has been shown to inhibit SUMO2 chain formation <ref name="ubcsumocomplex"/>. NMR studies of Ubc9 have demonstrated that noncovalent interactions between SUMO-1 and Ubc9 play a critical role in the transfer of SUMO-1 from the activating enzyme (E1) to the conjugating enzyme (E2) <ref name="noncovalent Nterminal">PMID:12924945</ref>. |
== Disease == | == Disease == | ||
Current revision
| |||||||||||
References
- ↑ 1.0 1.1 Tong H, Hateboer G, Perrakis A, Bernards R, Sixma TK. Crystal structure of murine/human Ubc9 provides insight into the variability of the ubiquitin-conjugating system. J Biol Chem. 1997 Aug 22;272(34):21381-7. PMID:9261152
- ↑ 2.0 2.1 Sekhri P, Tao T, Kaplan F, Zhang XD. Characterization of amino acid residues within the N-terminal region of Ubc9 that play a role in Ubc9 nuclear localization. Biochem Biophys Res Commun. 2015 Feb 27;458(1):128-33. doi:, 10.1016/j.bbrc.2015.01.081. Epub 2015 Jan 27. PMID:25637535 doi:http://dx.doi.org/10.1016/j.bbrc.2015.01.081
- ↑ Yunus AA, Lima CD. Lysine activation and functional analysis of E2-mediated conjugation in the SUMO pathway. Nat Struct Mol Biol. 2006 Jun;13(6):491-9. Epub 2006 May 28. PMID:16732283 doi:http://dx.doi.org/10.1038/nsmb1104
- ↑ Reverter D, Lima CD. Insights into E3 ligase activity revealed by a SUMO-RanGAP1-Ubc9-Nup358 complex. Nature. 2005 Jun 2;435(7042):687-92. PMID:15931224 doi:10.1038/nature03588
- ↑ 5.0 5.1 5.2 5.3 Knipscheer P, van Dijk WJ, Olsen JV, Mann M, Sixma TK. Noncovalent interaction between Ubc9 and SUMO promotes SUMO chain formation. EMBO J. 2007 Jun 6;26(11):2797-807. Epub 2007 May 10. PMID:17491593
- ↑ Gupta MK, Gulick J, Liu R, Wang X, Molkentin JD, Robbins J. Sumo E2 enzyme UBC9 is required for efficient protein quality control in cardiomyocytes. Circ Res. 2014 Sep 26;115(8):721-9. doi: 10.1161/CIRCRESAHA.115.304760. Epub 2014, Aug 5. PMID:25097219 doi:http://dx.doi.org/10.1161/CIRCRESAHA.115.304760
- ↑ Tatham MH, Chen Y, Hay RT. Role of two residues proximal to the active site of Ubc9 in substrate recognition by the Ubc9.SUMO-1 thiolester complex. Biochemistry. 2003 Mar 25;42(11):3168-79. PMID:12641448 doi:http://dx.doi.org/10.1021/bi026861x
- ↑ Lee B, Muller MT. SUMOylation enhances DNA methyltransferase 1 activity. Biochem J. 2009 Jul 15;421(3):449-61. doi: 10.1042/BJ20090142. PMID:19450230 doi:http://dx.doi.org/10.1042/BJ20090142
- ↑ Kulinski A, Rustaeus S, Vance JE. Microsomal triacylglycerol transfer protein is required for lumenal accretion of triacylglycerol not associated with ApoB, as well as for ApoB lipidation. J Biol Chem. 2002 Aug 30;277(35):31516-25. Epub 2002 Jun 18. PMID:12072432 doi:http://dx.doi.org/10.1074/jbc.M202015200
- ↑ Tatham MH, Kim S, Yu B, Jaffray E, Song J, Zheng J, Rodriguez MS, Hay RT, Chen Y. Role of an N-terminal site of Ubc9 in SUMO-1, -2, and -3 binding and conjugation. Biochemistry. 2003 Aug 26;42(33):9959-69. PMID:12924945 doi:http://dx.doi.org/10.1021/bi0345283
- ↑ Kumar A, Ito A, Hirohama M, Yoshida M, Zhang KY. Identification of sumoylation inhibitors targeting a predicted pocket in Ubc9. J Chem Inf Model. 2014 Oct 27;54(10):2784-93. doi: 10.1021/ci5004015. Epub 2014, Sep 18. PMID:25191977 doi:http://dx.doi.org/10.1021/ci5004015
- ↑ Mo YY, Moschos SJ. Targeting Ubc9 for cancer therapy. Expert Opin Ther Targets. 2005 Dec;9(6):1203-16. PMID:16300471 doi:http://dx.doi.org/10.1517/14728222.9.6.1203
- ↑ Li H, Niu H, Peng Y, Wang J, He P. Ubc9 promotes invasion and metastasis of lung cancer cells. Oncol Rep. 2013 Apr;29(4):1588-94. doi: 10.3892/or.2013.2268. Epub 2013 Feb 1. PMID:23381475 doi:http://dx.doi.org/10.3892/or.2013.2268