We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
UBC13 MMS2
From Proteopedia
(Difference between revisions)
| (3 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | <Structure load='1J7D' size=' | + | <Structure load='1J7D' size='350' scene='69/695700/Ubc13_mms2_overall/1' caption='Human ubiquitin conjugating enzyme E2 13 complex with HMM2 (PDB code [[1j7d]])' > |
==Summary== | ==Summary== | ||
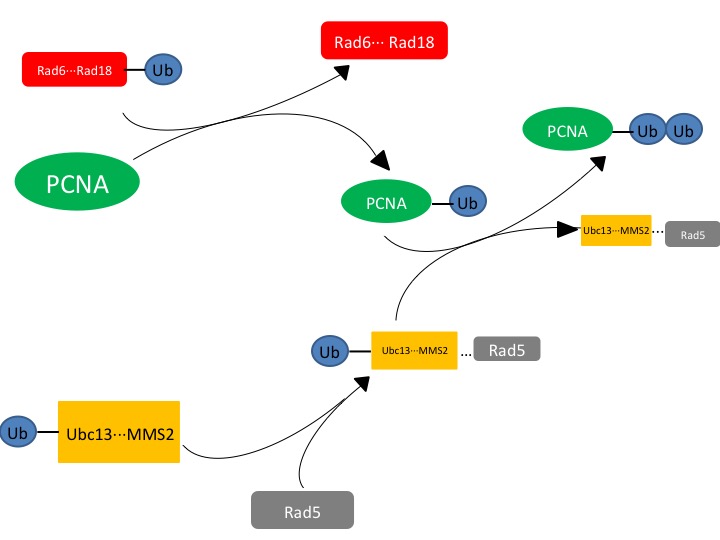
| - | Ubc13 is an E2 ubiquitin-conjugating enzyme that can form a heterodimer with Mms2 to function as a part of the translesion synthesis (TLS) pathway,<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref><ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. When bound to Mms2, Ubc13 will polyubiquitinate proliferating cell nuclear antigen (PCNA), a sliding clamp protein at the DNA transcription fork<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref>. Ubc13-Mms2 functions to polyubiquitinate PCNA following the initial monoubiquitination by Rad6-Rad18 (another E2 complex)<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. It is important to note that Ubc13 lacks the ability to be catalytically active without Mms2, hinting at inaccuracies within the statement "structure determines function." | + | '''Ubc13''' is an '''E2 ubiquitin-conjugating enzyme''' that can form a heterodimer with Mms2 to function as a part of the translesion synthesis (TLS) pathway,<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref><ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. When bound to Mms2, Ubc13 will polyubiquitinate proliferating cell nuclear antigen (PCNA), a sliding clamp protein at the DNA transcription fork<ref name=halas>3. Halas, A.; Podlaska, A. F.; Derkacz, J. F.; McIntyre, J. F.; Skoneczna, A. F.; Sledziewska-Gojska, E. The roles of PCNA SUMOylation, Mms2-Ubc13 and Rad5 in translesion DNA synthesis in Saccharomyces cerevisiae. Molecular microbiology JID - 8712028 0809.</ref>. Ubc13-Mms2 functions to polyubiquitinate PCNA following the initial monoubiquitination by Rad6-Rad18 (another E2 complex)<ref name=anderson>1. Andersen, P. L.; Zhou, H. F.; Pastushok, L. F.; Moraes, T. F.; McKenna, S. F.; Ziola B FAU - Ellison, Michael,J.; FAU, E. M.; FAU, D. V.; Xiao, W. Distinct regulation of Ubc13 functions by the two ubiquitin-conjugating enzyme variants Mms2 and Uev1A. The Journal of cell biology JID - 0375356 1107.</ref>. It is important to note that Ubc13 lacks the ability to be catalytically active without Mms2, hinting at inaccuracies within the statement "structure determines function." |
== Function == | == Function == | ||
| Line 36: | Line 36: | ||
The exact mechanism for how Ubc13 transfers ubiquitin is not known, however the mechanism occurs in either a step-wise or concerted reaction. Ubc13, as an E2, froms a covalent bond with ubiquitin and then transfers the ubiquitin to the target protein via a thioester intermediate. Ubiquitin is removed from Ubc13 and Mms2 complex and placed onto PCNA. | The exact mechanism for how Ubc13 transfers ubiquitin is not known, however the mechanism occurs in either a step-wise or concerted reaction. Ubc13, as an E2, froms a covalent bond with ubiquitin and then transfers the ubiquitin to the target protein via a thioester intermediate. Ubiquitin is removed from Ubc13 and Mms2 complex and placed onto PCNA. | ||
| + | ==3D structure of Ubc13== | ||
| + | [[Ubiquitin conjugating enzyme]] | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
Proteopedia Page Contributors and Editors (what is this?)
David A Taves, Michal Harel, Christopher Alexander Hudson, Nicholas R. Dunham