We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
BAG protein
From Proteopedia
(Difference between revisions)
| (14 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
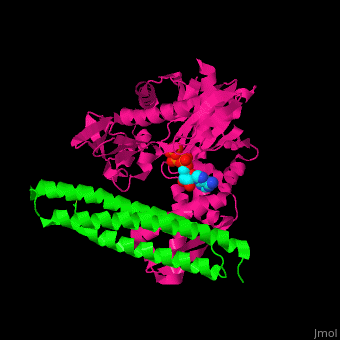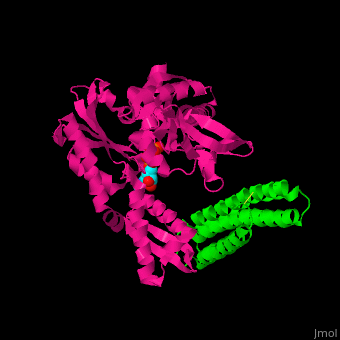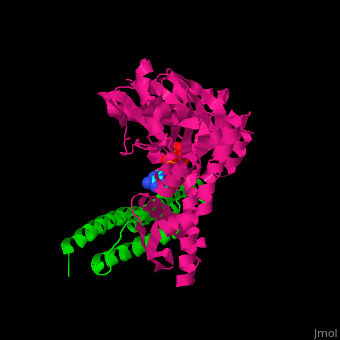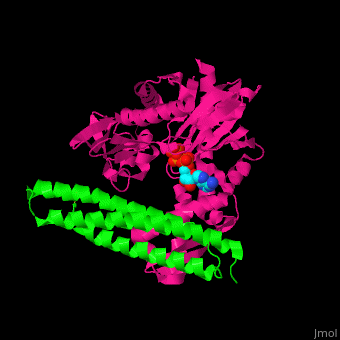
| - | <StructureSection load=' | + | <StructureSection load='' size='350' side='right' caption='Structure of human BAG-1 BAG domain (green) complex with HSC70 ATPase domain (magenta) and ATP (PDB entry [[3fzf]])' scene='56/568986/Cv/1'> |
| + | |||
| + | == Function == | ||
| - | The '''BAG family proteins''' ('''Bcl-2 associated athanogenes''') perform diverse functions. | + | The '''BAG family proteins''' ('''Bcl-2 associated athanogenes''') or '''BAG-family molecular chaperone protein''' perform diverse functions. '''BAG-1, BAG-2, BAG-4, BAG-5''' or '''BAG family molecular chaperone regulator''' inhibit the chaperone function of HSC70 and have anti-apoptotic function. <ref>PMID:18264803</ref> |
| - | </ | + | |
| - | == | + | == Structural highlights == |
| - | + | BAG proteins contain a common BAG domain ca. 45 amino acid long near the C terminal which is conserved. | |
| - | + | ||
| - | + | ==3D structures of BAG family proteins== | |
| + | [[BAG family proteins 3D structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | == References == | |
| - | + | <references/> | |
| - | + | ||
[[Category: Topic Page]] | [[Category: Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Kabbage M, Dickman MB. The BAG proteins: a ubiquitous family of chaperone regulators. Cell Mol Life Sci. 2008 May;65(9):1390-402. doi: 10.1007/s00018-008-7535-2. PMID:18264803 doi:http://dx.doi.org/10.1007/s00018-008-7535-2