We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Ferritin
From Proteopedia
(Difference between revisions)
| (30 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
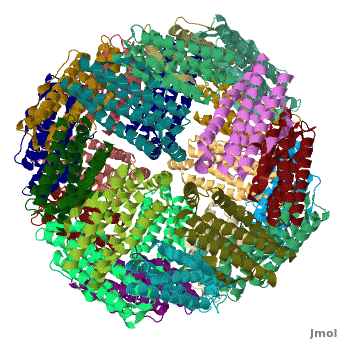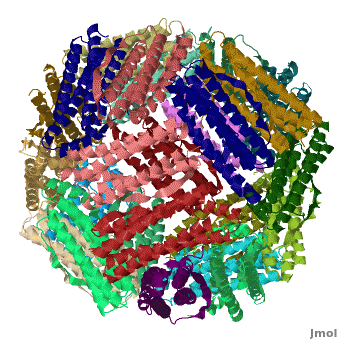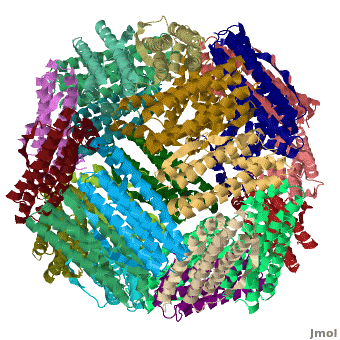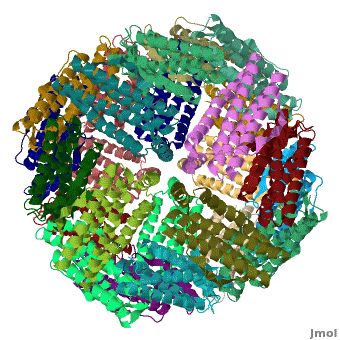
| - | < | + | <StructureSection load='' size='400' side='right' caption="Crystal structure of Fe-soaked ferritin from the hyperthermophilic archael anaerobe ''Pyrococcus furiousus'' [[2jd7]]" scene='Ferritin/Starting_ferritin_scene/1'> |
| - | [[Ferritin]] (FR) is an iron storage and release protein. It stores iron as microcrystals with phosphate and hydroxide ions. FR is composed of 24 subunits of heavy chain (FTH) and light chain (FTL).<br /> | ||
| - | * '''Bacterioferritin''' (BFR) structure is very similar to FR. It contains a binuclear iron center and haem. It stores iron as ferric oxide mineral in its hollow central cavity.<br /> | ||
| - | * '''Thioferritin''' (TFR) is a ferritin-related protein with 2 cysteine residues adjacent to the dimetal binding site.<br /> | ||
| - | * Another iron storing protein is the '''DNA-binding Protein of Starved cells''' (Dps) - a ferritin-like diiron carboxylate.<br /> | ||
| - | * '''MrgA''' – another iron storage protein - belongs to the Dps family. | ||
| - | == | + | == Function == |
| - | + | [[Ferritin]] (FR) is an iron storage and release protein. It stores iron as microcrystals with phosphate and hydroxide ions. FR is composed of 24 subunits of heavy chain (FTH) and light chain (FTL).<ref>PMID:20304033</ref> Amphibians have an additional middle subunit FR (FTM).<br /> | |
| - | + | * '''apo-ferritin''' (apo-FR) is a non-Fe-containing ferritin.<br /> | |
| + | * '''Bacterioferritin''' (BFR) structure is very similar to FR. It contains a binuclear iron center and haem. It stores iron as ferric oxide mineral in its hollow central cavity<ref>PMID:37375226</ref>.<br /> | ||
| + | * '''Thioferritin''' (TFR) is a ferritin-related protein with 2 cysteine residues adjacent to the dimetal binding site.<br /> | ||
| + | * '''DNA-binding Protein of Starved cells''' (Dps) - a ferritin-like diiron carboxylate which acts as a molecular chaperone and may confer thermotolerance in ''E. coli''<ref>PMID:37229826</ref>.<br /> | ||
| - | + | == Relevance == | |
| - | + | Cavities formed by FR are used for the manufacture of nanoparticles. FR is used as a marker for iron overload disorder. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | == Disease == | |
| - | + | FR deficiency can lead to anemia. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ==3D Structures of Ferritin== | |
| - | + | [[Ferritin 3D structures]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | </StructureSection> | |
| - | **[[3iq1]] – VcDps <br /> | ||
| - | **[[3ge4]] – BmDps <br /> | ||
| - | **[[2iy4]] – Dps + Fe – ''Listeria monocytogenes''<br /> | ||
| - | **[[2bkc]], [[2bk6]], [[2bjy]] – LiDps (mutant) <br /> | ||
| - | **[[2f7n]] – Dps + Co – ''Deinococcus radiodurans''<br /> | ||
| - | **[[4a25]] - Dps + Mn – ''Kinecoccus radiodurans''<br /> | ||
| - | **[[1tjo]], [[1tk6]], [[1tkp]], [[1tko]], [[1moj]] – Dps + Fe – ''Halobacterium salinarum''<br /> | ||
| - | **[[1o9r]] – Dps + Fe – ''Agrobacterium tumefaciens''<br /> | ||
| - | **[[1dps]], [[1f33]] – EcDps <br /> | ||
| - | **[[1f30]] – EcDps + Zn<br /> | ||
| - | **[[1jre]] – EcDps + Cd<br /> | ||
| - | **[[1jts]], [[1l8h]], [[1l8i]] – EcDps (mutant)<br /> | ||
| - | **[[2ux1]] – SsDps + Zn – ''Streptococcus suis''<br /> | ||
| - | **[[2v15]] – SsDps + Te<br /> | ||
| - | **[[2xjm]] – SsDps + Co<br /> | ||
| - | **[[2xjn]] – SsDps + Cu<br /> | ||
| - | **[[2xjo]] – SsDps + Ni<br /> | ||
| - | **[[2xkq]] – SsDps + Mn<br /> | ||
| - | **[[2yw6]], [[2yw7]] – MsDps<br /> | ||
| - | **[[3ak8]], [[3ak9]] – Dps + Mg – ''Salmonella enterica''<br /> | ||
| - | **[[1n1q]] – Dps + Fe2O– ''Brevibacillus brevis''<br /> | ||
| - | **[[1vei]], [[1vel]], [[1veq]] – MsDps + Fe <br /> | ||
| - | **[[4dyu]] – Dpo + Zn – ''Yersinia pestis'' | ||
| - | + | == References == | |
| + | <references/> | ||
| - | **[[2chp]] – MrgA – ''Bacillus subtilis''<br /> | ||
| - | }} | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Wang W, Knovich MA, Coffman LG, Torti FM, Torti SV. Serum ferritin: Past, present and future. Biochim Biophys Acta. 2010 Aug;1800(8):760-9. doi: 10.1016/j.bbagen.2010.03.011. , Epub 2010 Mar 19. PMID:20304033 doi:http://dx.doi.org/10.1016/j.bbagen.2010.03.011
- ↑ van der Ven AM, Gyamfi H, Suttisansanee U, Ahmad MS, Su Z, Taylor RM, Poole A, Chiorean S, Daub E, Urquhart T, Honek JF. Molecular Engineering of E. coli Bacterioferritin: A Versatile Nanodimensional Protein Cage. Molecules. 2023 Jun 9;28(12):4663. PMID:37375226 doi:10.3390/molecules28124663
- ↑ Park JH, Lee ES, Jung YJ. Functional characterization of the DNA-binding protein from starved cells (DPS) as a molecular chaperone under heat stress. Biochem Biophys Res Commun. 2023 Jul 30;667:180-185. PMID:37229826 doi:10.1016/j.bbrc.2023.05.064
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Joel L. Sussman, David Canner, Eran Hodis, Alexander Berchansky