Fibrinogen
From Proteopedia
(Difference between revisions)
m |
|||
| (8 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='1n73' size='350' side='right' scene='41/410324/Cv/1' caption='Crystal structure of glycosylated fibrinogen fragment D. Subunit α (green and yellow), β (green and magenta), γ (pink and cyan) complex with the peptide ligand Gly-His-Arg-Pro-amide (red, wheat, blue, black) and Ca+2 ion (PDB code [[1n73]]) '> | |
| + | |||
| + | == Function == | ||
| + | |||
| + | '''Fibrinogen''' is a glycoprotein found in the blood that is converted into fibrin during blood coagulation. Fibrinogen is cleaved by another protein, [[thrombin]], exposing knobs A and B to form fibrin. <ref>PMID:16689770</ref> The fibrin forms clots to prevent excessive bleeding from wounds sustained. Clotting factors, like factor XIII, are often linked to fibrin. <ref>PMID:18673233</ref> | ||
| + | |||
| + | ==3D Printed Physical Model of Fibrinogen== | ||
| + | |||
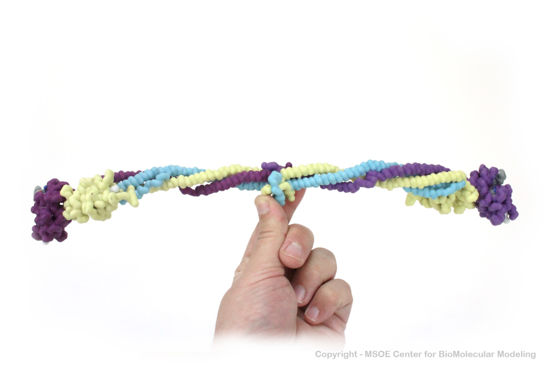
| + | Shown below is a 3D printed physical model of Fibrinogen. The structure is shown as an alpha carbon backbone colored by chain, with the three chains of each copy of fibrinogen colored yellow, blue and purple. | ||
| + | |||
| + | [[Image:fibrinogen1_centerForBioMolecularModeling.jpg|550px]] | ||
| + | |||
| + | |||
| + | ====The MSOE Center for BioMolecular Modeling==== | ||
| + | |||
| + | [[Image:CbmUniversityLogo.jpg | left | 150px]] | ||
| + | |||
| + | The [http://cbm.msoe.edu MSOE Center for BioMolecular Modeling] uses 3D printing technology to create physical models of protein and molecular structures, making the invisible molecular world more tangible and comprehensible. To view more protein structure models, visit our [http://cbm.msoe.edu/educationalmedia/modelgallery/ Model Gallery]. | ||
| + | |||
| + | |||
| + | == Structural insights == | ||
| - | '''Fibrinogen''' is a glycoprotein found in the blood that is converted into fibrin during blood coagulation. Fibrinogen is cleaved by another protein, [[thrombin]], exposing knobs A and B to form fibrin. <ref>PMID:16689770</ref> It is this fibrin that forms clots to prevent excessive bleeding from wounds sustained. Clotting factors, like factor XIII, are often linked to fibrin. <ref>PMID:18673233</ref> | ||
Fibrinogen is composed of 2 copies each of 3 non-identical chains α, β, γ (<scene name='Fibrinogen/Fba/1'>Fba</scene>, <scene name='Fibrinogen/Fbb/1'>Fbb</scene>, <scene name='Fibrinogen/Fbg/1'>Fbg</scene>). | Fibrinogen is composed of 2 copies each of 3 non-identical chains α, β, γ (<scene name='Fibrinogen/Fba/1'>Fba</scene>, <scene name='Fibrinogen/Fbb/1'>Fbb</scene>, <scene name='Fibrinogen/Fbg/1'>Fbg</scene>). | ||
| + | </StructureSection> | ||
==3D Structure of Fibrinogen== | ==3D Structure of Fibrinogen== | ||
| Line 16: | Line 36: | ||
**[[2a45]] - hFba+hFbb+hFbg+thrombin+PPACK thrombin inhibitor<br /> | **[[2a45]] - hFba+hFbb+hFbg+thrombin+PPACK thrombin inhibitor<br /> | ||
**[[1re4]], [[1rf0]], [[1n8e]], [[1lt9]] - hFba+hFbb+hFbg<br /> | **[[1re4]], [[1rf0]], [[1n8e]], [[1lt9]] - hFba+hFbb+hFbg<br /> | ||
| - | **[[1jy3]], [[1deq]] – | + | **[[1jy3]], [[1deq]] – bFba+Fbb+Fbg – bovine<br /> |
| - | **[[1jy2]] - | + | **[[1jy2]] - bFba+Fbb+Fbg proteolytic fragment<br /> |
| + | **[[1m1j]] - cFba+Fbb+Fbg+peptide ligand – chicken<br /> | ||
| + | **[[1ei3]] - cFba+Fbb+Fbg<br /> | ||
**[[1n73]], [[1lwu]] - Fba+Fbb+Fbg+peptide ligand – Sea lamprey<br /> | **[[1n73]], [[1lwu]] - Fba+Fbb+Fbg+peptide ligand – Sea lamprey<br /> | ||
| - | **[[1m1j]] - heFba+heFbb+heFbg+peptide ligand – hen<br /> | ||
| - | **[[1ei3]] - heFba+heFbb+heFbg<br /> | ||
*Fibrinogen α chain | *Fibrinogen α chain | ||
| Line 26: | Line 46: | ||
**[[1fzd]] – hFba EC domain<br /> | **[[1fzd]] – hFba EC domain<br /> | ||
**[[1bbr]] – hFba+cε-thrombin<br /> | **[[1bbr]] – hFba+cε-thrombin<br /> | ||
| - | **[[2jor]] – | + | **[[2jor]] – bFba – NMR<br /> |
| - | **[[2baf]] – | + | **[[2baf]] – bFba<br /> |
*Fibrinogen β chain | *Fibrinogen β chain | ||
| Line 36: | Line 56: | ||
*Fibrinogen γ chain | *Fibrinogen γ chain | ||
| + | **[[1fic]], [[1fid]] – hFbg<br /> | ||
| + | **[[3fib]] – hFbg C terminal<br /> | ||
**[[2vr3]] – hFbg+SaClumping Factor A – ''Staphylococcus aureus''<br /> | **[[2vr3]] – hFbg+SaClumping Factor A – ''Staphylococcus aureus''<br /> | ||
| - | **[[2vdo]], [[2vdp]], [[2vdq]], [[2vdr]] – hFbg+Integrin alpha IIB+Integrin beta-3+ | + | **[[2vdo]], [[2vdp]], [[2vdq]], [[2vdr]] – hFbg+Integrin alpha IIB+Integrin beta-3+ antibody <br /> |
**[[2fib]], [[3fib]] - hFbg+peptide ligand<br /> | **[[2fib]], [[3fib]] - hFbg+peptide ligand<br /> | ||
**[[1fib]] – hFbg+Ca<br /> | **[[1fib]] – hFbg+Ca<br /> | ||
| - | **[[1fic]], [[1fid]] – hFbg<br /> | ||
| - | **[[3fib]] – hFbg C terminal<br /> | ||
| - | **[[1dug]] – Fbg C-terminal/glutathione S-transferase – ''Schistosoma japonicum''<br /> | ||
**[[2hwl]] – hFbg peptide+prothrombin<br /> | **[[2hwl]] – hFbg peptide+prothrombin<br /> | ||
**[[2y7l]] – hFbg + agglutinin-like protein <br /> | **[[2y7l]] – hFbg + agglutinin-like protein <br /> | ||
| + | **[[1dug]] – Fbg C-terminal/glutathione S-transferase – ''Schistosoma japonicum''<br /> | ||
}} | }} | ||
==References== | ==References== | ||
| Line 50: | Line 70: | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
| + | [[Category:3D printer files]] | ||
Current revision
| |||||||||||
3D Structure of Fibrinogen
Updated on 28-November-2025
References
- ↑ Betts L, Merenbloom BK, Lord ST. The structure of fibrinogen fragment D with the 'A' knob peptide GPRVVE. J Thromb Haemost. 2006 May;4(5):1139-41. PMID:16689770 doi:10.1111/j.1538-7836.2006.01902.x
- ↑ Muszbek L, Bagoly Z, Bereczky Z, Katona E. The involvement of blood coagulation factor XIII in fibrinolysis and thrombosis. Cardiovasc Hematol Agents Med Chem. 2008 Jul;6(3):190-205. PMID:18673233
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, David Canner, Mark Hoelzer, Alexander Berchansky, Marius Mihasan, Angel Herraez