Sandbox Reserved 1063
From Proteopedia
(Difference between revisions)
| (303 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | = | + | =='''Adhesin Competence Regulator (AdcR)'''== |
| - | <StructureSection load=' | + | <StructureSection load='3TGN' size='350' side='right' caption='[http://www.rcsb.org/pdb/explore/explore.do?structureId=3TGN Adhesin Competence Regulator (3TGN)]' scene=''> |
| - | == Introduction == | + | ===Introduction=== |
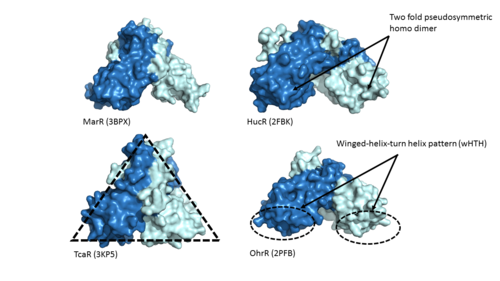
| - | + | Adhesin Competence Regulator (<scene name='69/694230/Adcr_space_fill/1'>AdcR</scene>) is a transcriptional regulator that controls the activation of over seventy genes within the bacterium [https://en.wikipedia.org/wiki/Streptococcus_pneumoniae''Streptococcus pneumoniae''] <ref name="Sanson">DOI:10.1093/nar/gku1304 </ref> and is a member of the multiple antibiotic resistance regulator (MarR) protein family <ref> PMID: 23428319</ref>. Members of the Mar R protein family conserve a number of features including a general triangular shape, a two fold pseudosymmetric homodimer, and a winged helix-turn-helix pattern [https://en.wikipedia.org/wiki/Helix-turn-helix (wHTH)] which can be seen in Figure 1. AdcR exhibits these conserved features as well, while also exhibiting its own distinct features. | |
| - | + | ||
| - | + | [[Image:MarR_protein_family_slide.png|500px|left|thumb|'''Figure 1: MarR protein family features'''. Proteins MarR [http://www.rcsb.org/pdb/explore/explore.do?structureId=3bpx (3BPX)], HucR [http://www.rcsb.org/pdb/explore/explore.do?structureId=2FBK (2FBK)], TcaR [http://www.rcsb.org/pdb/explore/explore.do?structureId=3KP5 (3KP5)], and OhrR [http://www.rcsb.org/pdb/explore/explore.do?structureId=2pfb (2PFB)] are pictured above with conserved features of the MarR protein family highlighted]] | |
| - | '' | + | In contrast with other members of the MarR family, AdcR is metal dependent. Zinc plays a vital role in organism homeostasis, acting as a [https://en.wikipedia.org/wiki/Cofactor_(biochemistry) co-factor] and a regulator of enzymatic activity. However zinc can lead to cell toxicity and deficiency of other vital metals that are also necessary for protein function <ref> DOI: 10.1021/cr900077w</ref>. Binding of Zinc allows AdcR to bind DNA and activate the transcription of high-affinity Zinc specific uptake transporters. The importance of AdcR in ''Streptococcus pneumoniae'' can be understood provided its ability to regulate zinc transfer proteins within the bacteria. |
| - | == Structural | + | ==='''Structural Overview'''=== |
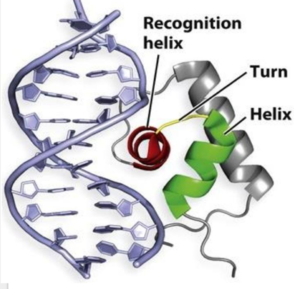
| - | + | [[Image:Screen Shot 2017-04-18 at 11.56.32 PM.png|300 px|right|thumb|'''Figure 2'''. A generic protein representing the [https://images.google.com/imgres?imgurl=https%3A%2F%2Fclassconnection.s3.amazonaws.com%2F838%2Fflashcards%2F2220838%2Fjpg%2Fasdf-144940F88BA53A918F3.jpg&imgrefurl=https%3A%2F%2Fwww.studyblue.com%2Fnotes%2Fnote%2Fn%2Flecture-13%2Fdeck%2F10226974&docid=qBvv1vgKeLTGcM&tbnid=3nuaRjPWKUBfqM%3A&vet=1&w=741&h=756&hl=en&source=sh%2Fx%2Fim wHTH] motif binding the major and minor groove of DNA similar to AdcR.]] | |
| - | + | One of the two functional domains of AdcR is the <scene name='69/694230/Dimerization_domain/3'> dimerization domain</scene>. This domain connects and stabilizes the two pseudosymmetric protomers and is composed of the <scene name='69/694230/Alpha_1/1'>α1 helix</scene>, the <scene name='69/694230/Alpha_6/1'>α6 helix</scene> . and the C-terminus of the <scene name='69/694230/Alpha_five/1'>α5 helix</scene> . This domain is connected to the [https://en.wikipedia.org/wiki/DNA-binding_domain DNA binding domain] by the long α5 helix. The DNA binding domain interacts with the major and minor grooves of DNA via the <scene name='69/694230/Whth_4/7'>winged helix-turn-helix (wHTH)</scene> motif (Figure 2). The binding of Zinc to the <scene name='69/694230/2_binding_sites/4'>Zinc binding pocket</scene> induces a conformational change that allows for a <scene name='69/694230/Hydrogen_bonding_1/4'>hydrogen bond network</scene> between 4 specific residues. This network connects multiple helices from the metal binding pockets and DNA binding domain, and is believed play a critical role in the allosteric activation of AdcR, allowing the protein to bind exposed bases along the major and minor grooves of the DNA ligand <ref name="guerra">PMID:22085181</ref>. Thus, the protein is able to perform its biological function by activating transcription after binding DNA. | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | The FadD13 enzyme functions to activate lipids. Once the lipids are activated, they can continue on into metabolic pathways. This is done by ATP/AMP binding to the <scene name='69/694230/Fadd13_subunits/12'>ATP/AMP binding region</scene>. Once ATP/AMP is bound, the long lipid chain up to 26 carbons may bind in the <scene name='69/694230/Fadd13_subunits/13'>hydrophobic tunnel</scene> of the enzyme. Upon binding of the substrate, the C terminal swings up to close off the tunnel. From there CoA can bind to produce the final product, an acyl-CoA Thioester. The lipid can now move transversely throughout the membrane and throughout the rest of the cell. Figure 2 shows the proposed mechanism for ACSVL proteins<ref name="Anderson 2012"/>. | ||
| - | == | + | == '''DNA Binding''' == |
| - | + | === Helix-Turn-Helix Motif === | |
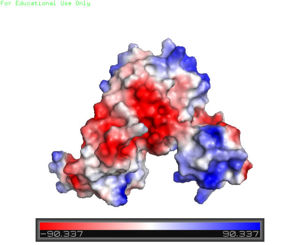
| + | [[Image:Charge_map.jpg |300 px|right|thumb|'''Figure 3'''. A charge map of AdcR shows the general triangular shape and the <font color='blue'>positively</font> charged area on the tips of the wHTH motif]] | ||
| + | The AdcR MarR transcriptional regulator's structure resembles that of other proteins in the MarR family; however, the most notable differences are found in the winged helix-turn-helix (wHTH) motif that assists in binding DNA <ref name="guerra" />. The <scene name='69/694230/Whth_4/7'>winged helix turn helix</scene> motif is made up of the <font color='blue'>α3</font> and <font color='blue'>α4 helices</font> along with <scene name='69/694230/Anti-parallel_beta_sheet/2'>anti-parallel β sheets</scene> on each side. There is one wHTH motif per monomer. The recognition helix, or the α4 helix, binds the major groove of DNA through [https://en.wikipedia.org/wiki/Hydrogen_bond hydrogen bonding] and [https://en.wikipedia.org/wiki/Van_der_Waals_force Van der Waals interactions] between exposed bases <ref name="guerra" />. The wings of the helix bind the minor groove of DNA while the other helices stabilize the DNA and Protein upon binding. The two anti parallel β sheets contain several <scene name='69/694230/Positive_residues_on_wing_3/4'>Arginine, Asparagine, and Lysine residues</scene> that stabilize this interaction between DNA. The charge map (Figure 3) highlights the dark blue tips of the wHTH motif consisting of lysine and arginine residues, which stabilize the negatively charged backbone of DNA. The residues are only shown on the random loop of one monomer because the random loop on the other protein monomer <scene name='69/694230/Uncrystallized_loop/1'>was not crystallized</scene>. | ||
| + | |||
| + | == '''Zn(II) Binding''' == | ||
| + | Zinc-Dependent Transcriptional Regulator AdcR has <scene name='69/694230/2_binding_sites/4'>two binding sites for zinc</scene> on each of its two protomers and can bind a total of four Zn(II) ions. The <scene name='69/694230/Alpha1-alpha2_loop/2'>α1-α2 loop</scene> combined with the <scene name='69/694230/Alpha_five/1'>α5 helix</scene> and <scene name='69/694230/Alpha_2/1'>α2 helix</scene> contribute residues to the <scene name='69/694230/Two_binding_sites/2'>metal binding sites</scene>. Each protomer has one high affinity site (Binding site 1; KZn<sub>1</sub> = 10<sup>12</sup> M; pH 8) and one low affinity binding site (Binding Site 2; KZn<sub>2</sub> = 10<sup>7</sup> M; pH 8) <ref name="Reyes">PMID:20804771</ref>. The two different Zn(II) binding sites are connected via <scene name='69/694230/Hydrogen_bonding/5'>hydrogen bonding</scene> of H108 and E41. | ||
| + | === Binding Site 1 === | ||
| + | <scene name='69/694230/Binding_site_1/5'>Binding site 1</scene> consists of a distorted tetrahedral geometry around Zn(II). The four amino acids involved in zinc binding are E24, H42, H108, and H112. Binding site 1 is the only binding site that plays a significant role in the protein's regulatory function. The ability of binding site 1 to coordinate to the Zn(II) ion is pH dependent. At pH 6 the binding affinity for the Zn(II) ion is 10<sup>9</sup> - 10<sup>10</sup> M<sup>-1</sup>, but at pH 8 the binding affinity increases to 10<sup>12</sup> M<sup>-1</sup> <ref name="Reyes" />. This is due to the charges on the histidines of the binding site. At pH 6, the histidines are positively charged and are not able to interact with the positively charged Zn(II) ion. However, at pH 8 the histidines are neutrally charged and are able to coordinate with Zn(II), which increases the overall binding affinity. The AdcR MarR transcriptional regulator is able to bind Co(II) in binding site 1 in a way that induces similar conformational changes to Zn(II) binding. Co(II) coordination in binding site 1 is able to allosterically activate DNA binding similarly to Zn(II) binding <ref name="guerra" />. | ||
| + | |||
| + | === Binding Site 2 === | ||
| + | <scene name='69/694230/Binding_site_2/4'>Binding site 2</scene> consists of a highly distorted tetrahedral geometry around the zinc ion. There are three amino acids involved in the binding of the zinc ion (C30, E41, and E107) as well as a water molecule (shown as a red sphere). When Cys30 in binding site 2 is mutated to an alanine, it has no effect on the ability of the protein to bind DNA <ref name="guerra" />. Therefore, binding site 2 has no significant role in the ability of AdcR to bind to DNA and AdcR is still able to function with no zinc bound present in binding site 2. In fact, the presence of binding site 2 may simply be due to an excess of zinc during the crystallization process. | ||
| + | |||
| + | === Hydrogen Bond Network === | ||
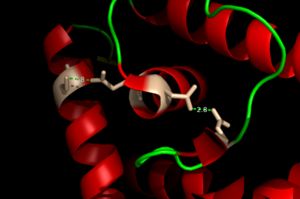
| + | [[Image:H Bonding of DNA.png|300 px|left|thumb|'''Figure 4'''. The Hydrogen Bonding Network is shown with dotted green lines approximately 2.8 angstroms between residues.]] | ||
| + | The binding of zinc metals creates a hydrogen bond network (Figure 4) within the protein that connects the metal binding sites and the [https://en.wikipedia.org/wiki/DNA-binding_domain DNA binding domain]. The <scene name='69/694230/Hydrogen_bonding_1/5'>hydrogen bond network</scene> (<scene name='69/694230/Hydrogen_bonding_2/5'>with measurements</scene>) (residues in stick structures, colored by atom type) is characteristic of the MarR family as a whole and connects the metal binding pockets to the α4 helix also known as the DNA recognition helix. <scene name='69/694230/Recognition_helix/3'>Several residues</scene> in this helix recognize the DNA ligand. The hydrogen bond network connects the α2 and α4 helices via hydrogen bonding between specific residues. After zinc is bound, a glutamate (E24) residue from a random coil accepts a hydrogen bond from the carboxamide end of an asparagine (N38) residue from the α2 helix. A glutamine (Q40) residue from α2 helix accepts a hydrogen bond from a serine (S74) residue from the α4 helix <ref name="guerra" />. | ||
| + | |||
| + | |||
| + | == '''Clinical Relevance''' == | ||
| + | ''Streptococcus pneumoniae'' is a significant pathogenic bacterium. Although asymptomatic in healthy individuals, ''S. pneumoniae'' can lead to Bronchitis, meningitis conjunctivitis, or brain abscesses in those with weaker immune systems. Host regulation of zinc is often used to combat pathogens such as ''S. pneumoniae'' <ref name="Sanson" />. A better understanding of AdcR, the regulator that controls the transcription of zinc specific uptake transporters, could help to illuminate better mechanism for combating not only ''S. pneumoniae'', but other comparable bacteria. | ||
| - | ==Relevant Pages== | ||
| - | * [http://proteopedia.org/wiki/index.php/3t5b 3T5B] | ||
| - | * [http://proteopedia.org/wiki/index.php/3t5c 3T5C] | ||
</StructureSection> | </StructureSection> | ||
| - | == References == | + | |
| - | + | ||
| + | == References == | ||
| + | <references/> | ||
Current revision
Adhesin Competence Regulator (AdcR)
| |||||||||||
References
- ↑ 1.0 1.1 Sanson M, Makthal N, Flores AR, Olsen RJ, Musser JM, Kumaraswami M. Adhesin competence repressor (AdcR) from Streptococcus pyogenes controls adaptive responses to zinc limitation and contributes to virulence. Nucleic Acids Res. 2015 Jan;43(1):418-32. doi: 10.1093/nar/gku1304. Epub 2014 Dec, 15. PMID:25510500 doi:http://dx.doi.org/10.1093/nar/gku1304
- ↑ Grove A. MarR family transcription factors. Curr Biol. 2013 Feb 18;23(4):R142-3. doi: 10.1016/j.cub.2013.01.013. PMID:23428319 doi:http://dx.doi.org/10.1016/j.cub.2013.01.013
- ↑ Ma Z, Jacobsen FE, Giedroc DP. Coordination chemistry of bacterial metal transport and sensing. Chem Rev. 2009 Oct;109(10):4644-81. doi: 10.1021/cr900077w. PMID:19788177 doi:http://dx.doi.org/10.1021/cr900077w
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Guerra AJ, Dann CE, Giedroc DP. Crystal Structure of the Zinc-Dependent MarR Family Transcriptional Regulator AdcR in the Zn(II)-Bound State. J Am Chem Soc. 2011 Nov 21. PMID:22085181 doi:10.1021/ja2080532
- ↑ 5.0 5.1 Reyes-Caballero H, Guerra AJ, Jacobsen FE, Kazmierczak KM, Cowart D, Koppolu UM, Scott RA, Winkler ME, Giedroc DP. The metalloregulatory zinc site in Streptococcus pneumoniae AdcR, a zinc-activated MarR family repressor. J Mol Biol. 2010 Oct 22;403(2):197-216. doi: 10.1016/j.jmb.2010.08.030. Epub 2010, Sep 8. PMID:20804771 doi:http://dx.doi.org/10.1016/j.jmb.2010.08.030