We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Sandbox WWC4
From Proteopedia
(Difference between revisions)
| (One intermediate revision not shown.) | |||
| Line 1: | Line 1: | ||
==Aquaporin (AQP) The Water Channel of the cell== | ==Aquaporin (AQP) The Water Channel of the cell== | ||
<StructureSection load='1h6i' size='350' side='right' caption='Aquaporin' scene='Aquaporin'> | <StructureSection load='1h6i' size='350' side='right' caption='Aquaporin' scene='Aquaporin'> | ||
| + | <scene name='69/696849/Hydrophobic_and_polar/1'>1H6I hydrophobic and polar</scene> | ||
Aquqporin (AQP) is a integral membrane proteins that control the amount of water that travels in and out of the cell<ref name="Takata">PMID:15242101</ref>. Before the discovery of aquaporin by Peter Agre (1992); it was thought that water molecules leaked through the phospholipid bilayer one by one. However this could not be the case for cells that need to released and uptake water quickly like in the kidneys. | Aquqporin (AQP) is a integral membrane proteins that control the amount of water that travels in and out of the cell<ref name="Takata">PMID:15242101</ref>. Before the discovery of aquaporin by Peter Agre (1992); it was thought that water molecules leaked through the phospholipid bilayer one by one. However this could not be the case for cells that need to released and uptake water quickly like in the kidneys. | ||
Aquaporins allow water to flow rapidly to the inside of cell then it would by crossing the bilayer.<ref name="Takata" /> The presence of water channels increases membrane permeability to water<ref name="Takata" />. This protein is highly selective to water molecules and prevents the passage of ions and other solutes.There are multiple types of aquaporins in the human boby, some can allow the transport of other molecules such as glycerol,CO2, ammonia and urea. For example aquaporin 3 has a pore width of 8-10 Ångströms and allows the passage of hydrophilic molecules ranging between 150-200 Da<ref name="Takata" />. | Aquaporins allow water to flow rapidly to the inside of cell then it would by crossing the bilayer.<ref name="Takata" /> The presence of water channels increases membrane permeability to water<ref name="Takata" />. This protein is highly selective to water molecules and prevents the passage of ions and other solutes.There are multiple types of aquaporins in the human boby, some can allow the transport of other molecules such as glycerol,CO2, ammonia and urea. For example aquaporin 3 has a pore width of 8-10 Ångströms and allows the passage of hydrophilic molecules ranging between 150-200 Da<ref name="Takata" />. | ||
| + | |||
| + | [[Image:AQP-Figure-3-Full.gif|300px|left|thumb|Water Orientation in AQP]] | ||
| Line 10: | Line 13: | ||
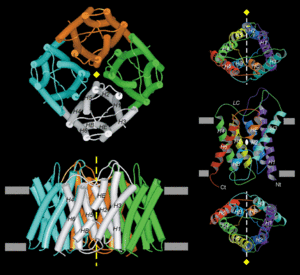
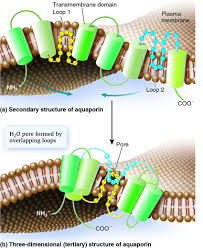
AQP1 monomer contains 269 amino acid residues, which form two tandem repeats of three membrane-spanning α-helices.Aquaporin is a protien with six transmembrane alpha helices with the amino and craboxyl terminal tails located in the cytoplasm.<ref name="Murata">PMID:11034202</ref>. Water molecules traverse through the pore of the channel in single file<ref name="Takata" />.Each monomer is able to channel water.There is a conserved Asn-Pro-Ala sequence that overlap in the middle of the lipid bilayer membrane which creates trail for the water molecules to be carried along in the channel. | AQP1 monomer contains 269 amino acid residues, which form two tandem repeats of three membrane-spanning α-helices.Aquaporin is a protien with six transmembrane alpha helices with the amino and craboxyl terminal tails located in the cytoplasm.<ref name="Murata">PMID:11034202</ref>. Water molecules traverse through the pore of the channel in single file<ref name="Takata" />.Each monomer is able to channel water.There is a conserved Asn-Pro-Ala sequence that overlap in the middle of the lipid bilayer membrane which creates trail for the water molecules to be carried along in the channel. | ||
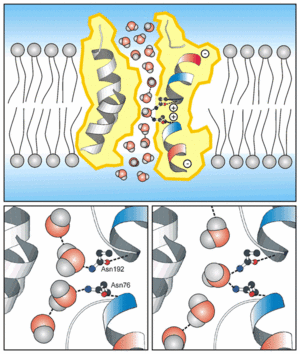
| - | The hydrogen-bonding interactions of water molecules with the polar side chains of Asn-76 and Asn-192 on the strictly conserved Asn-Pro-Ala sequence motifs were found to be essential for maintaining the connectivity of water flow in the narrow constriction region<ref name="Kong">PMID:PMC64684</ref>. | + | The hydrogen-bonding interactions of water molecules with the polar side chains of Asn-76 and Asn-192 on the strictly conserved Asn-Pro-Ala sequence motifs were found to be essential for maintaining the connectivity of water flow in the narrow constriction region<ref name="Kong">PMID:PMC64684</ref>. |
| + | |||
| + | [[Image:AQP-Figure-4-Full.gif|300px|right|thumb|Water Orientation in AQP]] | ||
The hourglass shape allows the water flows, these water pores are completely impermeable to charged species, such as protons. Which is very important to the conservation of membrane's electrochemical potential<ref name="Takata" />. | The hourglass shape allows the water flows, these water pores are completely impermeable to charged species, such as protons. Which is very important to the conservation of membrane's electrochemical potential<ref name="Takata" />. | ||
| Line 39: | Line 44: | ||
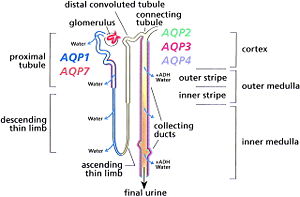
In the Kidney, they are found in the basolateral and apical plasma membranes of the proximal tubules and the limb of the loop of Henle in the kidney.Aquaporin are concentrated in the kidney is where there is a need to transporting large amounts of water in and out of the cell.<ref name="Cardiac" /> | In the Kidney, they are found in the basolateral and apical plasma membranes of the proximal tubules and the limb of the loop of Henle in the kidney.Aquaporin are concentrated in the kidney is where there is a need to transporting large amounts of water in and out of the cell.<ref name="Cardiac" /> | ||
| - | [[Image:F2.large.jpg|300px| | + | [[Image:F2.large.jpg|300px|right|thumb|Water Orientation in AQP]] |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
Current revision
Aquaporin (AQP) The Water Channel of the cell
| |||||||||||