Sandbox WWCAlpha-S1-Casein
From Proteopedia
| (One intermediate revision not shown.) | |||
| Line 3: | Line 3: | ||
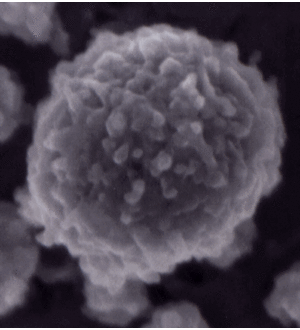
[[Image:Micelle.gif|300px|right|thumb|Casein micelle image from Dalgleish, D. G., P. Spagnuolo and H. D. Goff. 2004. A possible structure of the casein micelle based on high-resolution field-emission scanning electron microscopy. International Dairy Journal. 14: 1025-1031. This micelle is 120 nm in diameter.<ref>https://www.uoguelph.ca/foodscience/book-page/structure-casein-micelle</ref>]] | [[Image:Micelle.gif|300px|right|thumb|Casein micelle image from Dalgleish, D. G., P. Spagnuolo and H. D. Goff. 2004. A possible structure of the casein micelle based on high-resolution field-emission scanning electron microscopy. International Dairy Journal. 14: 1025-1031. This micelle is 120 nm in diameter.<ref>https://www.uoguelph.ca/foodscience/book-page/structure-casein-micelle</ref>]] | ||
== History == | == History == | ||
| - | All caseins are in the secretory calcium-binding phosphoprotein (SCPP) family and share odontogenic ameloblast–associated (ODAM) protein as a common ancestor.<ref name="Kawasaki & Lafont" /> ODAM is a SCPP involved in the mineralization of teeth, and has a primary structure rich in proline and glutamine. The caseins share this P/Q richness, giving the proteins a flexible, open structure <ref>Kawasaki K, Buchanan AV, Weiss KM. 2007. Gene duplication and the evolution of vertebrate skeletal mineralization. Cells Tissues Organs. 186:7–24.</ref> | + | All caseins are in the secretory calcium-binding phosphoprotein (SCPP) family and share odontogenic ameloblast–associated (ODAM) protein as a common ancestor.<ref name="Kawasaki & Lafont" /> ODAM is a SCPP involved in the mineralization of teeth, and has a primary structure rich in proline and glutamine. The caseins share this P/Q richness, giving the proteins a flexible, open structure. <ref>Kawasaki K, Buchanan AV, Weiss KM. 2007. Gene duplication and the evolution of vertebrate skeletal mineralization. Cells Tissues Organs. 186:7–24.</ref> |
== Biological Function == | == Biological Function == | ||
Calcium-sensitive (CS) caseins such as αs1-casein bind calcium phosphate (CaP), and are precipitated by high concentrations of CaP. In milk, large complexes are formed from CaP, calcium-sensitive caseins, and calcium-insensitive (CI) caseins. Precipitation of the CS caseins, which occurs at millimolar concentrations of ionic calcium, is prevented by CI caseins, stabilizing the complex to form micelle 50-500 nm in diameter.<ref>Phadungath, C. (2005). Casein micelle structure: a concise review. Songklanakarin Journal of Science and Technology, 27(1), 201-212.</ref> In addition to providing nutritive protein to mammalian neonates, these micelles supply calcium and inorganic phosphate at levels much higher than would be expected if the molecules were simply dissolved in the milk.<ref>McSweeney, P. (2009). Nutritional Aspects of Milk Proteins. In Advanced dairy chemistry (3rd ed., Vol. 1). New York: Springer-Verlag.</ref> The caseins in milk also work to prevent pathalogical calcification of mammary tissue.<ref>Holt C. Casein structure and casein–calcium phosphate interactions. In: Proceedings of 25th International Dairy Congress, Aarhus, Denmark. Copenhagen: Danish National Committee of International Dairy Federation, 1998:200–208</ref> Unlike whey proteins, which include all non-casein proteins, casein micelles are relatively heat stable, although denaturation of the whey protein β-lactoglobulin at high temperatures can result in interactions with micellar κ-casein that alters the structure of the micelle surface.<ref>Fennema, O. (1996). Characteristics of Milk. In Food chemistry (3rd ed., p. 865). New York: Marcel Dekker.</ref> | Calcium-sensitive (CS) caseins such as αs1-casein bind calcium phosphate (CaP), and are precipitated by high concentrations of CaP. In milk, large complexes are formed from CaP, calcium-sensitive caseins, and calcium-insensitive (CI) caseins. Precipitation of the CS caseins, which occurs at millimolar concentrations of ionic calcium, is prevented by CI caseins, stabilizing the complex to form micelle 50-500 nm in diameter.<ref>Phadungath, C. (2005). Casein micelle structure: a concise review. Songklanakarin Journal of Science and Technology, 27(1), 201-212.</ref> In addition to providing nutritive protein to mammalian neonates, these micelles supply calcium and inorganic phosphate at levels much higher than would be expected if the molecules were simply dissolved in the milk.<ref>McSweeney, P. (2009). Nutritional Aspects of Milk Proteins. In Advanced dairy chemistry (3rd ed., Vol. 1). New York: Springer-Verlag.</ref> The caseins in milk also work to prevent pathalogical calcification of mammary tissue.<ref>Holt C. Casein structure and casein–calcium phosphate interactions. In: Proceedings of 25th International Dairy Congress, Aarhus, Denmark. Copenhagen: Danish National Committee of International Dairy Federation, 1998:200–208</ref> Unlike whey proteins, which include all non-casein proteins, casein micelles are relatively heat stable, although denaturation of the whey protein β-lactoglobulin at high temperatures can result in interactions with micellar κ-casein that alters the structure of the micelle surface.<ref>Fennema, O. (1996). Characteristics of Milk. In Food chemistry (3rd ed., p. 865). New York: Marcel Dekker.</ref> | ||
| Line 11: | Line 11: | ||
== Structural highlights == | == Structural highlights == | ||
As rheomorphic proteins, caseins do not have a defined globular structure.<ref>Horne, D. S. (2002). Casein structure, self-assembly and gelation. Current opinion in colloid & interface science, 7(5), 456-461. doi:10.1016/S1359-0294(02)00082-1</ref> X-Ray crystallography at the level of secondary structure is therefore challenging and may be impossible, as sufficient electron density can not be obtained in crystals. The computer-generated "crystal" structure of bovine β-casein is shown in the scene to the left. As predicted by Phyre, 87% of β-casein is predicted disordered, and therefore there is very low confidence for its structure. | As rheomorphic proteins, caseins do not have a defined globular structure.<ref>Horne, D. S. (2002). Casein structure, self-assembly and gelation. Current opinion in colloid & interface science, 7(5), 456-461. doi:10.1016/S1359-0294(02)00082-1</ref> X-Ray crystallography at the level of secondary structure is therefore challenging and may be impossible, as sufficient electron density can not be obtained in crystals. The computer-generated "crystal" structure of bovine β-casein is shown in the scene to the left. As predicted by Phyre, 87% of β-casein is predicted disordered, and therefore there is very low confidence for its structure. | ||
| - | However, this is not to say that β-casein and others in the casein family are nonfunctional. As described above, the caseins function to take up calcium and phosphate in micelles. There are now many known similarly rheomorphic proteins, which use their ''lack'' of structure to perform their function. | + | |
| + | However, this is not to say that β-casein and others in the casein family are nonfunctional. As described above, the caseins function to take up calcium and phosphate in micelles. There are now many known similarly rheomorphic proteins, which use their ''lack'' of structure to perform their function. The primary structure for both CS and CI caseins is important for aggregation, and for the CI caseins enables stabilization of the casein micelle in the presence of calcium. Aggregation occurs mainly through binding at hydrophobic P- and Q-rich regions, and the CI caseins stabilize the micelle through relatively hydrophilic S- and T-rich regions at the micelle surface. | ||
| + | <ref name="Kawasaki & Lafont" /> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
Caseins consist of a family of phosphoproteins found in all mammal milks, which includes αs1-, αs2-, and β-casein (calcium-sensitive caseins), κ-casein (calcium insensitive).[1] Although αs1-casein is the predominant casein in bovine milk, the ratios of caseins vary considerably by species, and in human milk β-casein is predominant.[2][3]
Contents |
History
All caseins are in the secretory calcium-binding phosphoprotein (SCPP) family and share odontogenic ameloblast–associated (ODAM) protein as a common ancestor.[2] ODAM is a SCPP involved in the mineralization of teeth, and has a primary structure rich in proline and glutamine. The caseins share this P/Q richness, giving the proteins a flexible, open structure. [5]
Biological Function
Calcium-sensitive (CS) caseins such as αs1-casein bind calcium phosphate (CaP), and are precipitated by high concentrations of CaP. In milk, large complexes are formed from CaP, calcium-sensitive caseins, and calcium-insensitive (CI) caseins. Precipitation of the CS caseins, which occurs at millimolar concentrations of ionic calcium, is prevented by CI caseins, stabilizing the complex to form micelle 50-500 nm in diameter.[6] In addition to providing nutritive protein to mammalian neonates, these micelles supply calcium and inorganic phosphate at levels much higher than would be expected if the molecules were simply dissolved in the milk.[7] The caseins in milk also work to prevent pathalogical calcification of mammary tissue.[8] Unlike whey proteins, which include all non-casein proteins, casein micelles are relatively heat stable, although denaturation of the whey protein β-lactoglobulin at high temperatures can result in interactions with micellar κ-casein that alters the structure of the micelle surface.[9]
Preceding the formation of micelles, CS casein readily binds to CaP to form CaP nanoclusters. It is believed that the rheomorphic (non-rigid) structure of CS caseins is what allows such efficient CaP binding.[10] On average 800 CaP nanoclusters are present in a casein micelle[11], but this can vary considerably by species as the size of the micelle changes.
|
Structural highlights
As rheomorphic proteins, caseins do not have a defined globular structure.[12] X-Ray crystallography at the level of secondary structure is therefore challenging and may be impossible, as sufficient electron density can not be obtained in crystals. The computer-generated "crystal" structure of bovine β-casein is shown in the scene to the left. As predicted by Phyre, 87% of β-casein is predicted disordered, and therefore there is very low confidence for its structure.
However, this is not to say that β-casein and others in the casein family are nonfunctional. As described above, the caseins function to take up calcium and phosphate in micelles. There are now many known similarly rheomorphic proteins, which use their lack of structure to perform their function. The primary structure for both CS and CI caseins is important for aggregation, and for the CI caseins enables stabilization of the casein micelle in the presence of calcium. Aggregation occurs mainly through binding at hydrophobic P- and Q-rich regions, and the CI caseins stabilize the micelle through relatively hydrophilic S- and T-rich regions at the micelle surface. [2]
References
- ↑ J. Dairy Sci., 67, 1599-1631, 1984, and from Table 1, J. Dairy Sci., 68, 2195-2205, 1985
- ↑ 2.0 2.1 2.2 Kawasaki K, Lafont AG, Sire JY. The evolution of milk casein genes from tooth genes before the origin of mammals. Mol Biol Evol. 2011 Jul;28(7):2053-61. doi: 10.1093/molbev/msr020. Epub 2011 Jan , 18. PMID:21245413 doi:http://dx.doi.org/10.1093/molbev/msr020
- ↑ McSweeney, P. (2009). Nutritional Aspects of Milk Proteins. In Advanced dairy chemistry (3rd ed., Vol. 1). New York: Springer-Verlag.
- ↑ https://www.uoguelph.ca/foodscience/book-page/structure-casein-micelle
- ↑ Kawasaki K, Buchanan AV, Weiss KM. 2007. Gene duplication and the evolution of vertebrate skeletal mineralization. Cells Tissues Organs. 186:7–24.
- ↑ Phadungath, C. (2005). Casein micelle structure: a concise review. Songklanakarin Journal of Science and Technology, 27(1), 201-212.
- ↑ McSweeney, P. (2009). Nutritional Aspects of Milk Proteins. In Advanced dairy chemistry (3rd ed., Vol. 1). New York: Springer-Verlag.
- ↑ Holt C. Casein structure and casein–calcium phosphate interactions. In: Proceedings of 25th International Dairy Congress, Aarhus, Denmark. Copenhagen: Danish National Committee of International Dairy Federation, 1998:200–208
- ↑ Fennema, O. (1996). Characteristics of Milk. In Food chemistry (3rd ed., p. 865). New York: Marcel Dekker.
- ↑ Uversky VN, Dunker AK. Understanding protein non-folding. Biochim Biophys Acta. 2010 Jun;1804(6):1231-64. doi:, 10.1016/j.bbapap.2010.01.017. Epub 2010 Feb 1. PMID:20117254 doi:http://dx.doi.org/10.1016/j.bbapap.2010.01.017
- ↑ Smyth E, Clegg RA, Holt C. A biological perspective on the structure and function of caseins and casein micelles. Int J Dairy Technol 2004;57:121-126.
- ↑ Horne, D. S. (2002). Casein structure, self-assembly and gelation. Current opinion in colloid & interface science, 7(5), 456-461. doi:10.1016/S1359-0294(02)00082-1
