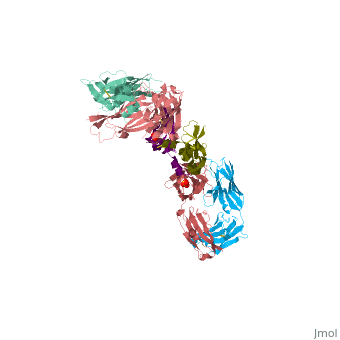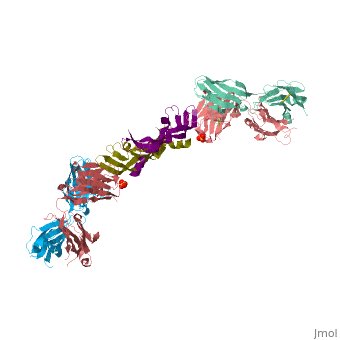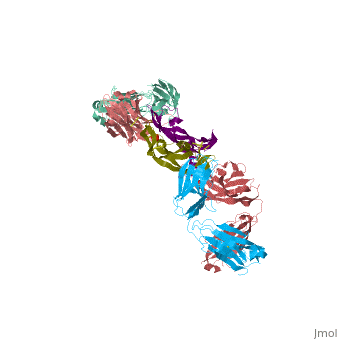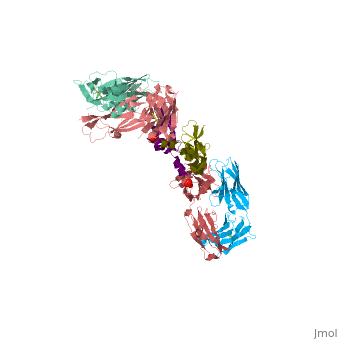VEGF IN COMPLEX WITH A NEUTRALIZING ANTIBODY
From Proteopedia
| (24 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | ==VEGF | + | <StructureSection load='1bj1' size='340' side='right' caption='Human VEGF receptor binding domain (dark olive, purple) complex with antibody (aqua, blue, red, rust) (PDB code [[1bj1]])' scene=''> |
| + | |||
| + | |||
| + | == Backround == | ||
'''Vascular endothelial growth factor''' ([http://en.wikipedia.org/wiki/Vascular_endothelial_growth_factor VEGF]), is a signal protein produced by cells that stimulates vasculogenesis and angiogenesis. It is part of the system that restores the oxygen supply to tissues when blood circulation is inadequate. | '''Vascular endothelial growth factor''' ([http://en.wikipedia.org/wiki/Vascular_endothelial_growth_factor VEGF]), is a signal protein produced by cells that stimulates vasculogenesis and angiogenesis. It is part of the system that restores the oxygen supply to tissues when blood circulation is inadequate. | ||
VEGF's normal function is to create new blood vessels during embryonic development, new blood vessels after injury, muscle following exercise, and new vessels (collateral circulation) to bypass blocked vessels. | VEGF's normal function is to create new blood vessels during embryonic development, new blood vessels after injury, muscle following exercise, and new vessels (collateral circulation) to bypass blocked vessels. | ||
| - | When VEGF is overexpressed, it can contribute to disease. Solid cancers cannot grow beyond a limited size without an adequate blood supply; cancers that can express VEGF are able to grow and metastasize. Overexpression of VEGF can cause vascular disease in the retina of the eye and other parts of the body. Drugs such as bevacizumab and ranibizumab can inhibit VEGF and control or slow those diseases. | + | When VEGF is overexpressed, it can contribute to disease. Solid cancers cannot grow beyond a limited size without an adequate blood supply; cancers that can express VEGF are able to grow and metastasize. Overexpression of VEGF can cause vascular disease in the retina of the eye and other parts of the body. Drugs such as bevacizumab and ranibizumab (both antibodies) can inhibit VEGF and control or slow those diseases. |
VEGF are important signaling proteins involved in both vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature). | VEGF are important signaling proteins involved in both vasculogenesis (the de novo formation of the embryonic circulatory system) and angiogenesis (the growth of blood vessels from pre-existing vasculature). | ||
| Line 12: | Line 15: | ||
'''Monoclonal antibodies''' (mAb or moAb) are monospecific antibodies that are made by identical immune cells that are all clones of a unique parent cell, in contrast to polyclonal antibodies which are made from several different immune cells. Monoclonal antibodies have monovalent affinity, in that they bind to the same epitope. | '''Monoclonal antibodies''' (mAb or moAb) are monospecific antibodies that are made by identical immune cells that are all clones of a unique parent cell, in contrast to polyclonal antibodies which are made from several different immune cells. Monoclonal antibodies have monovalent affinity, in that they bind to the same epitope. | ||
Given almost any substance, it is possible to produce monoclonal antibodies that specifically bind to that substance; they can then serve to detect or purify that substance. This has become an important tool in biochemistry, molecular biology and medicine. | Given almost any substance, it is possible to produce monoclonal antibodies that specifically bind to that substance; they can then serve to detect or purify that substance. This has become an important tool in biochemistry, molecular biology and medicine. | ||
| - | |||
| - | <StructureSection load='1bj1' size='340' side='right' caption='Caption for this structure' scene=''> | ||
| - | |||
| - | [[1bj1]] | ||
| - | |||
| - | <scene name='70/701451/Antibodies_fragment_chains/3'>VEGF antibodies chains</scene> | ||
| - | <ref>PMID 9753694</ref> | ||
| - | This is a default text for your page '''VEGF IN COMPLEX WITH A NEUTRALIZING ANTIBODY'''. Click above on '''edit this page''' to modify. Be careful with the < and > signs. | ||
| - | You may include any references to papers as in: the use of JSmol in Proteopedia <ref>DOI 10.1002/ijch.201300024</ref> or to the article describing Jmol <ref name='maor vegf'>PMID:21638687</ref> to the rescue. | ||
| - | |||
| - | == Backround == | ||
==Related Diseases == | ==Related Diseases == | ||
| + | Defects in VEGFA are a cause of susceptibility to microvascular complications of diabetes type 1 (MVCD1) . These are pathological conditions that develop in numerous tissues and organs as a consequence of diabetes mellitus. They include diabetic retinopathy, diabetic nephropathy leading to end-stage renal disease, and diabetic neuropathy. Diabetic retinopathy remains the major cause of new-onset blindness among diabetic adults. It is characterized by vascular permeability and increased tissue ischemia and angiogenesis. | ||
| + | for more info click ([http://omim.org/entry/603933 here]) | ||
== Structural highlights == | == Structural highlights == | ||
| - | == | + | VEGF and anti-VEGF Fab Complex is a 6 chain, 1050 amino acid structure with a sequence taken from Homo sapiens and Mus musculus. |
| + | For a guided tour on the structure components use[http://oca.weizmann.ac.il/oca-docs/fgij/fg.htm?mol=1BJ1 FirstGlance] | ||
| + | VEGF is a <scene name='70/701451/Homodimeric_vegf/1'>homodimeric</scene> protein who belongs to the family of cystine knot growth factors <ref>PMID:9753694</ref> . | ||
| + | while the 4 other chains belongs to the <scene name='70/701451/1bj1_fab_fragment_emphesys/1'>FAB fragment</scene>. | ||
| + | VEGF and anti-VEGF Fab Complex | ||
| + | The structure of the <scene name='70/701451/Receptor_binding_domain/1'>receptor-binding domain</scene> (residues 8–109) of VEGF consists of a central four- stranded β sheet that displays the characteristic cystine knot at one end and possesses a small hydrophobic core at the other end. | ||
| - | + | ==3D structure of VEGF== | |
| + | |||
| + | [[Vascular Endothelial Growth Factor]] | ||
</StructureSection> | </StructureSection> | ||
| + | == Publication Abstract from PubMed == | ||
| + | BACKGROUND: Vascular endothelial growth factor (VEGF) is a highly specific angiogenic growth factor; anti-angiogenic treatment through inhibition of receptor activation by VEGF might have important therapeutic applications in diseases such as diabetic retinopathy and cancer. A neutralizing anti-VEGF antibody shown to suppress tumor growth in an in vivo murine model has been used as the basis for production of a humanized version. RESULTS: We present the crystal structure of the complex between VEGF and the Fab fragment of this humanized antibody, as well as a comprehensive alanine-scanning analysis of the contact residues on both sides of the interface. Although the VEGF residues critical for antibody binding are distinct from those important for high-affinity receptor binding, they occupy a common region on VEGF, demonstrating that the neutralizing effect of antibody binding results from steric blocking of VEGF-receptor interactions. Of the residues buried in the VEGF-Fab interface, only a small number are critical for high-affinity binding; the essential VEGF residues interact with those of the Fab fragment, generating a remarkable functional complementarity at the interface. CONCLUSIONS: Our findings suggest that the character of antigen-antibody interfaces is similar to that of other protein-protein interfaces, such as ligand-receptor interactions; in the case of VEGF, the principal difference is that the residues essential for binding to the Fab fragment are concentrated in one continuous segment of polypeptide chain, whereas those essential for binding to the receptor are distributed over four different segments and span across the dimer interface. | ||
| + | |||
| + | VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface.,Muller YA, Chen Y, Christinger HW, Li B, Cunningham BC, Lowman HB, de Vos AM Structure. 1998 Sep 15;6(9):1153-67. PMID:9753694 | ||
| + | |||
| + | From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.<br> | ||
| + | </div> | ||
== References == | == References == | ||
<references/> | <references/> | ||
Current revision
| |||||||||||
Publication Abstract from PubMed
BACKGROUND: Vascular endothelial growth factor (VEGF) is a highly specific angiogenic growth factor; anti-angiogenic treatment through inhibition of receptor activation by VEGF might have important therapeutic applications in diseases such as diabetic retinopathy and cancer. A neutralizing anti-VEGF antibody shown to suppress tumor growth in an in vivo murine model has been used as the basis for production of a humanized version. RESULTS: We present the crystal structure of the complex between VEGF and the Fab fragment of this humanized antibody, as well as a comprehensive alanine-scanning analysis of the contact residues on both sides of the interface. Although the VEGF residues critical for antibody binding are distinct from those important for high-affinity receptor binding, they occupy a common region on VEGF, demonstrating that the neutralizing effect of antibody binding results from steric blocking of VEGF-receptor interactions. Of the residues buried in the VEGF-Fab interface, only a small number are critical for high-affinity binding; the essential VEGF residues interact with those of the Fab fragment, generating a remarkable functional complementarity at the interface. CONCLUSIONS: Our findings suggest that the character of antigen-antibody interfaces is similar to that of other protein-protein interfaces, such as ligand-receptor interactions; in the case of VEGF, the principal difference is that the residues essential for binding to the Fab fragment are concentrated in one continuous segment of polypeptide chain, whereas those essential for binding to the receptor are distributed over four different segments and span across the dimer interface.
VEGF and the Fab fragment of a humanized neutralizing antibody: crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface.,Muller YA, Chen Y, Christinger HW, Li B, Cunningham BC, Lowman HB, de Vos AM Structure. 1998 Sep 15;6(9):1153-67. PMID:9753694
From MEDLINE®/PubMed®, a database of the U.S. National Library of Medicine.
</div>