Sulfhydryl oxidase
From Proteopedia
(Difference between revisions)
| (9 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
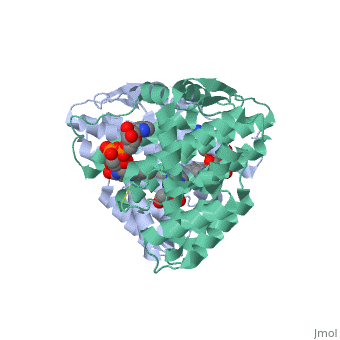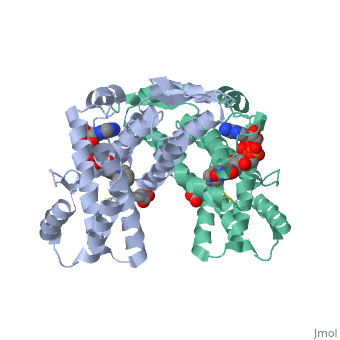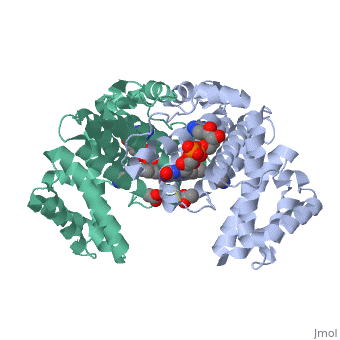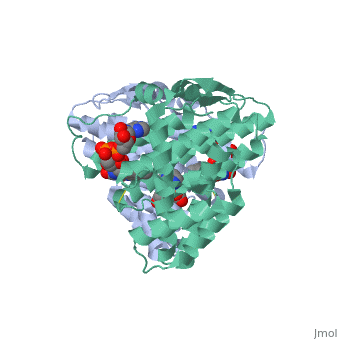
| - | + | <StructureSection load='3p0k' size='350' side='right' scene='' caption='Baculovirus sulfhydryl oxidase complex with acetate and imidazole, showing the FAD, [[3p0k]]'> | |
| - | '''Sulfhydryl oxidase''' (SOX) is a flavin-dependent enzyme which catalyze the formation of disulfide bonds from thiol groups. The reaction involves the reduction of O2 to hydrogen peroxide. Erv1p SOX is involved in the biogenesis of Fe/S clusters. ALR is a SOX augmenter of liver regeneration. QSOX is a quiescin SOX. QSOX contain thioredoxin domain, helix-rich domain and FAD-binding domain Erv/ALR. | ||
| - | + | '''Sulfhydryl oxidase''' (SOX) is a flavin-dependent enzyme which catalyzes the formation of disulfide bonds from thiol groups. The reaction involves the reduction of O<sub>2</sub> to hydrogen peroxide<ref>PMID:12176051</ref>. | |
| - | + | *'''Erv1p''' is involved in the biogenesis of Fe/S clusters<ref>PMID:10899311</ref>.<br /> | |
| - | + | *'''ALR''' is a SOX augmenter of liver regeneration<ref>PMID:23186364</ref>.<br /> | |
| + | *'''QSOX''' is a quiescin SOX. QSOX contains thioredoxin domain, helix-rich domain and FAD-binding domain Erv/ALR<ref>PMID:18393449</ref>. | ||
| - | * | + | *<scene name='46/468158/Cv/3'>FAD binding site</scene> in Baculovirus sulfhydryl oxidase ([[3p0k]]). Water molecules are shown as red spheres. |
| - | + | ==3D structures of sulfhydryl oxidase== | |
| - | + | [[Sulfhydryl oxidase 3D structures]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | </StructureSection> | |
| - | + | == References == | |
| - | + | <references/> | |
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Thorpe C, Hoober KL, Raje S, Glynn NM, Burnside J, Turi GK, Coppock DL. Sulfhydryl oxidases: emerging catalysts of protein disulfide bond formation in eukaryotes. Arch Biochem Biophys. 2002 Sep 1;405(1):1-12. PMID:12176051
- ↑ Lee J, Hofhaus G, Lisowsky T. Erv1p from Saccharomyces cerevisiae is a FAD-linked sulfhydryl oxidase. FEBS Lett. 2000 Jul 14;477(1-2):62-6. PMID:10899311
- ↑ Sztolsztener ME, Brewinska A, Guiard B, Chacinska A. Disulfide bond formation: sulfhydryl oxidase ALR controls mitochondrial biogenesis of human MIA40. Traffic. 2013 Mar;14(3):309-20. doi: 10.1111/tra.12030. Epub 2012 Dec 16. PMID:23186364 doi:http://dx.doi.org/10.1111/tra.12030
- ↑ Heckler EJ, Alon A, Fass D, Thorpe C. Human quiescin-sulfhydryl oxidase, QSOX1: probing internal redox steps by mutagenesis. Biochemistry. 2008 Apr 29;47(17):4955-63. doi: 10.1021/bi702522q. Epub 2008 Apr, 5. PMID:18393449 doi:http://dx.doi.org/10.1021/bi702522q