We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Adhesin
From Proteopedia
(Difference between revisions)
(New page: <StructureSection load='4f8p' size='340' side='right' caption='Adhesin PsaA complex with galactose (PDB ID 4f8p)' scene=''> '''Adhesins''' (Adh) are surface components of bacteria whi...) |
|||
| (26 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
| - | + | <StructureSection load='' size='350' side='right' caption='Adhesin PsaA complex with tert-butyl formate and galactose (stick model) (PDB ID [[4f8p]])' scene='70/705684/Cv/1'> | |
| - | <StructureSection load=' | + | |
| - | + | ||
== Function == | == Function == | ||
| - | + | '''Adhesins''' (Adh) are surface components of bacteria which facillitate adhesion to surfaces or other cells. Adh are specific surface recognition protein and are regarded as virulence factors.<ref>PMID:11043979</ref> '''FimH''' is the ''E. coli'' adhesin which is part of the type 1 pili of the bacteria. The pili is composed of subunits '''FimF, FimG''' and '''FimD'''<ref>PMID:18369105</ref>. The main Adhesins of the pathogen ''Mycoplasma genitalium'' are '''P110''' or '''Mgp-operon protein 3''' and '''P140'''<ref>PMID:30367053</ref>. See also [[Journal:Acta Cryst D:S2059798320008116|Novel structure of the N-terminal helical domain of BibA, a Group B Streptococcus immunogenic bacterial adhesin]]. | |
| - | + | For trimeric autotransporter adhesin see [[EibD]]. | |
| - | == | + | == Disease == |
| - | + | Bacterial pathogens use adhesins as a major factor in adhesion-based virulence. Adhesins serve as vaccine targets since they are essential to infection<ref>PMID:10341176</ref>. | |
| - | + | == Structural highlights == | |
| - | + | ||
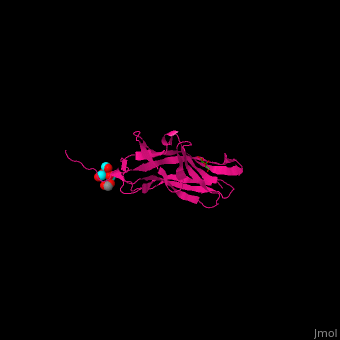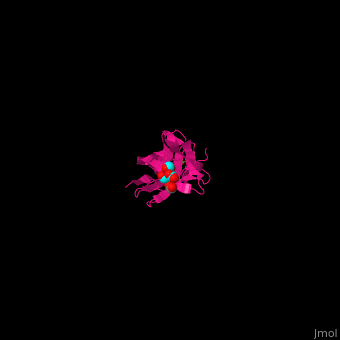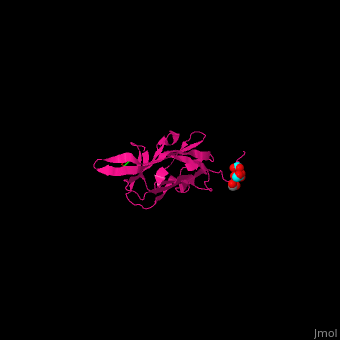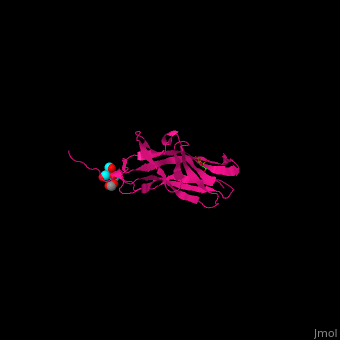
| - | * | + | <scene name='70/705684/Cv/4'>Adhesin PsaA complex with galactose, acetate and tert-butyl formate</scene> (PDB ID [[4f8p]])<ref>PMID:23277582</ref> |
| + | *<scene name='70/705684/Cv/3'>Galactose binding site</scene>. Water molecules are shown as red spheres. | ||
| + | *<scene name='70/705684/Cv/5'>Tert-butyl formate/Acetate binding site</scene>. | ||
| - | + | == 3D Structures of adhesin == | |
| - | + | [[Adhesin 3D structures]] | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | </StructureSection> | |
| - | **[[1amx]] – SaCAdh CBD – ''Staphylococcus aureus''<br /> | ||
| - | **[[1d2o]] – SaCAdh B1 repeat unit<br /> | ||
| - | **[[1d2p]] – SaCAdh B1-B2 repeat units<br /> | ||
| - | **[[2f68]] – SaCAdh extracellular domain<br /> | ||
| - | **[[2f6a]] – SaCAdh extracellular domain + collagen<br /> | ||
| - | **[[2okm]] – CAdh CBD – ''Enterococcus faecalis''<br /> | ||
| - | **[[1p9h]] – YeYadA CBD – ''Yersinia enterocolitica''<br /> | ||
| - | **[[3h7x]] – YeYadA stalk domain<br /> | ||
| - | **[[3h7z]] – YeYadA stalk domain (mutant)<br /> | ||
| - | **[[3lt6]], [[3lt7]] – YeYadA coiled coil<br /> | ||
| - | **[[2lme]] – YeYadA coiled coil - NMR<br /> | ||
| - | *Adhesin | ||
| - | |||
| - | **[[2odl]] – Adh secretion domain – ''Haemophilus influenzae''<br /> | ||
| - | }} | ||
| - | |||
| - | </StructureSection> | ||
== References == | == References == | ||
<references/> | <references/> | ||
| + | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ Klemm P, Schembri MA. Bacterial adhesins: function and structure. Int J Med Microbiol. 2000 Mar;290(1):27-35. PMID:11043979 doi:http://dx.doi.org/10.1016/S1438-4221(00)80102-2
- ↑ Nishiyama M, Ishikawa T, Rechsteiner H, Glockshuber R. Reconstitution of pilus assembly reveals a bacterial outer membrane catalyst. Science. 2008 Apr 18;320(5874):376-9. doi: 10.1126/science.1154994. Epub 2008 Mar, 27. PMID:18369105 doi:http://dx.doi.org/10.1126/science.1154994
- ↑ Aparicio D, Torres-Puig S, Ratera M, Querol E, Pinol J, Pich OQ, Fita I. Mycoplasma genitalium adhesin P110 binds sialic-acid human receptors. Nat Commun. 2018 Oct 26;9(1):4471. doi: 10.1038/s41467-018-06963-y. PMID:30367053 doi:http://dx.doi.org/10.1038/s41467-018-06963-y
- ↑ Wizemann TM, Adamou JE, Langermann S. Adhesins as targets for vaccine development. Emerg Infect Dis. 1999 May-Jun;5(3):395-403. doi: 10.3201/eid0503.990310. PMID:10341176 doi:http://dx.doi.org/10.3201/eid0503.990310
- ↑ Bao R, Nair MK, Tang WK, Esser L, Sadhukhan A, Holland RL, Xia D, Schifferli DM. Structural basis for the specific recognition of dual receptors by the homopolymeric pH 6 antigen (Psa) fimbriae of Yersinia pestis. Proc Natl Acad Sci U S A. 2013 Jan 15;110(3):1065-70. doi:, 10.1073/pnas.1212431110. Epub 2012 Dec 31. PMID:23277582 doi:10.1073/pnas.1212431110