We apologize for Proteopedia being slow to respond. For the past two years, a new implementation of Proteopedia has been being built. Soon, it will replace this 18-year old system. All existing content will be moved to the new system at a date that will be announced here.
Lipase
From Proteopedia
(Difference between revisions)
| (22 intermediate revisions not shown.) | |||
| Line 1: | Line 1: | ||
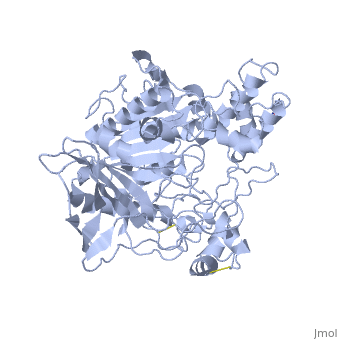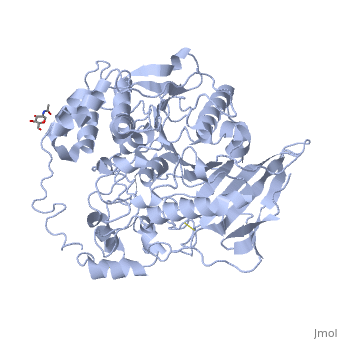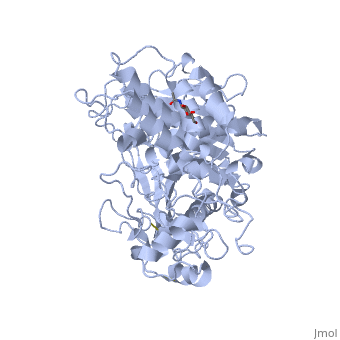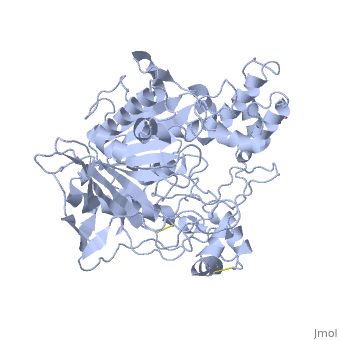
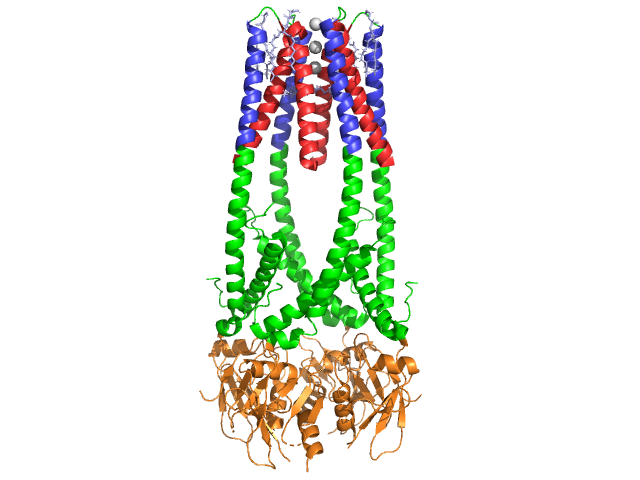
| - | <StructureSection load='1akn' size=' | + | <StructureSection load='1akn' size='300' side='right' caption='Structure of glycosylated pancreatic lipase (PDB entry [[1akn]])' scene=''> |
{{TOC limit|limit=2}} | {{TOC limit|limit=2}} | ||
== '''Introduction''' == | == '''Introduction''' == | ||
| - | '''Lipase''' catalyzes the breakdown of lipids by hydrolyzing the esters of fatty acids. Its function is important for digestion and promoting absorption of fats in the intestines. Lipase is primarily found in and secreted by the pancreas, but is also found in the saliva and stomach. | + | '''Lipase''' catalyzes the breakdown of lipids by hydrolyzing the esters of fatty acids. Its function is important for digestion and promoting absorption of fats in the intestines. Lipase is primarily found in and secreted by the pancreas, but is also found in the saliva and stomach.<br /> |
| + | * '''Pancreatic lipase''' (PDB ID: [[1hpl]]) which is pictured to the right, is a carboxylic ester hydrolase. It is also commonly called '''pancreatic triacylglycerol lipase''' and its enzyme class number is E.C. 3.1.1.3 <ref name="1HPL PDB SUM">[http://www.ebi.ac.uk/thornton-srv/databases/cgi-bin/pdbsum/GetPage.pl?pdbcode=1hpl&template=main.html] 1HPL PDB SUM </ref>.<br /> | ||
| + | * The '''bile salt-stimulated lipase''' (BSSL) is found in breast milk.<br /> | ||
| + | * The '''hormone-sensitive lipase''' (LIPE) hydrolyzes a variety of esters. For details see [[Hormone sensitive lipase]].<br /> | ||
| + | * '''Monoacylglycerol lipase''' (MAGL) hydrolyzes intracellular triglycerides to fatty acid and glycerol. MAGL functions together with LIPE. For details see [[Monoglyceride lipase]]. | ||
| + | |||
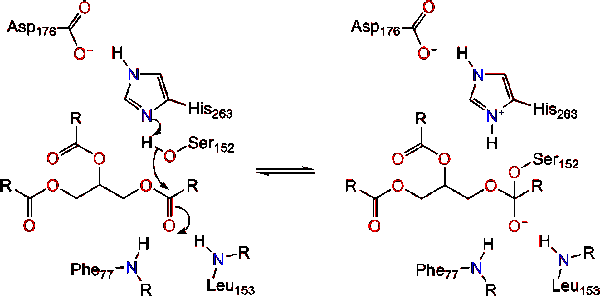
| + | The reaction catalyzed by the enzyme is shown below. | ||
[[Image:Picture 1.png]] | [[Image:Picture 1.png]] | ||
Further breakdown ultimately results in 2-monoacylglycerols and free fatty acids <ref name= "A cross-linked complex between horse pancreatic lipase and colipase">[http://www.sciencedirect.com/science/article/pii/0014579389815923] A cross-linked complex between horse pancreatic lipase and colipase</ref>. An in depth discussion of the mechanism can be found in the Lipase Catalytic Mechanism section. The determination of the structure and function of lipase was a gradual process. Lipase activity was first demonstrated in the pancreas by Claude Bernard in 1846. However, it wasn't until 1955 that Mattson and Beck demonstrated a high-specificity of pancreatic lipase for triglyceride primary esters <ref name= "History of Lipids">[http://www.cyberlipid.org/history/history1.htm] History of Lipids</ref>. In recent years, determination of the crystal structure of pancreatic lipase has become the primary focus as many scientists have worked to further this.<br /> | Further breakdown ultimately results in 2-monoacylglycerols and free fatty acids <ref name= "A cross-linked complex between horse pancreatic lipase and colipase">[http://www.sciencedirect.com/science/article/pii/0014579389815923] A cross-linked complex between horse pancreatic lipase and colipase</ref>. An in depth discussion of the mechanism can be found in the Lipase Catalytic Mechanism section. The determination of the structure and function of lipase was a gradual process. Lipase activity was first demonstrated in the pancreas by Claude Bernard in 1846. However, it wasn't until 1955 that Mattson and Beck demonstrated a high-specificity of pancreatic lipase for triglyceride primary esters <ref name= "History of Lipids">[http://www.cyberlipid.org/history/history1.htm] History of Lipids</ref>. In recent years, determination of the crystal structure of pancreatic lipase has become the primary focus as many scientists have worked to further this.<br /> | ||
| - | == | + | |
| + | ==See also== | ||
* [[Molecular Playground/Pancreatic Lipase]]<br /> | * [[Molecular Playground/Pancreatic Lipase]]<br /> | ||
* [[Lipase lid morph]]<br /> | * [[Lipase lid morph]]<br /> | ||
* [[Hormone sensitive lipase]]<br /> | * [[Hormone sensitive lipase]]<br /> | ||
| - | * [[Lipase from Candida antarctica in closed state]] | + | * [[Lipase from Candida antarctica in closed state]]<br /> |
| - | * [[Monoglyceride lipase]] | + | * [[Monoglyceride lipase]]<br /> |
| + | * [[Human gastric lipase]]<br /> | ||
| + | * [[Lipoprotein Lipase (LPL) complexed with GPIHBP1]]<br /> | ||
| + | * [[Lipase (Hebrew)]]<br /> | ||
| + | * [[Lipid metabolism]] | ||
== '''Structure''' == | == '''Structure''' == | ||
| Line 36: | Line 47: | ||
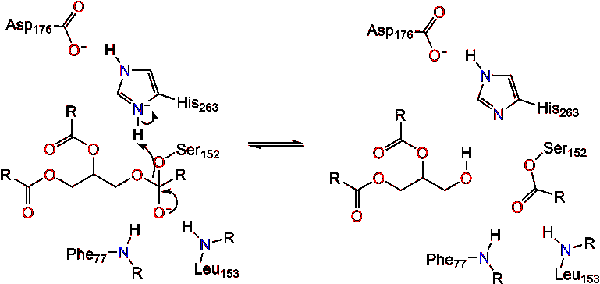
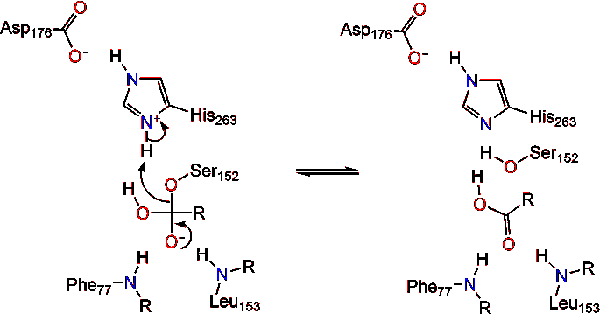
A water molecule then donates a proton to the histidine, creating a reactive hydroxyl anion. The hydroxyl anion can then attack the carbonyl carbon of the lipid, forming another negatively charged tetrahedral intermediate which is stabilized in the oxyanion hole (Reaction 3). | A water molecule then donates a proton to the histidine, creating a reactive hydroxyl anion. The hydroxyl anion can then attack the carbonyl carbon of the lipid, forming another negatively charged tetrahedral intermediate which is stabilized in the oxyanion hole (Reaction 3). | ||
| - | [[Image:M0218. | + | [[Image:M0218.stg03r.gif|center|]] |
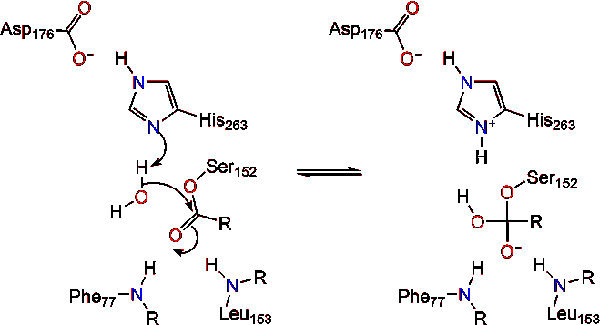
Upon reformation of the carbonyl, the catalytic serine is released and monoglyceride and fatty acid monomers diffuse away (Reaction 4). | Upon reformation of the carbonyl, the catalytic serine is released and monoglyceride and fatty acid monomers diffuse away (Reaction 4). | ||
| - | [[Image:M0218. | + | [[Image:M0218.stg04r.gif|center|]] |
== '''Inhibition of Pancreatic Lipase''' == | == '''Inhibition of Pancreatic Lipase''' == | ||
| Line 52: | Line 63: | ||
== '''Clinical Significance''' == | == '''Clinical Significance''' == | ||
Pancreatic lipase is secreted into the duodenum through the duct system of the pancreas. In a healthy individual, it is at very low concentration in serum. Under extreme disruption of pancreatic function, such as pancreatitis or pancreatic cancer, the pancreas may begin to digest itself and release pancreatic enzymes including pancreatic lipase into serum. Measurement of serum concentration of pancreatic lipase can therefore aid in diagnosis of acute pancreatitis.<ref>"Pancreatic lipase". Wikipedia: The Free Encyclopedia. 7 Nov 2011 [http://en.wikipedia.org/wiki/Pancreatic_lipase]</ref>. Due to lipase's activity in the digestion and absorption of fat, there has been a growing market for lipase inhibitors for weight loss pharmaceuticals. The most popular is Orlistat (or Xenical®) which is a natural product from ''Streptomyces toxytricini'' and is the hydrogenation product of lipostation- an irreversible lipase inhibitor. This inhibitor also acts by binding Ser152, producing an ester which hydrolyzes so slow that it is practically irreversible <ref>Kordik, C., Reitz, A. "Pharmacological Treatment of Obesity: Therapeutic Strategies" Journal of Medicinal Chemistry, 1999 (42).</ref>. | Pancreatic lipase is secreted into the duodenum through the duct system of the pancreas. In a healthy individual, it is at very low concentration in serum. Under extreme disruption of pancreatic function, such as pancreatitis or pancreatic cancer, the pancreas may begin to digest itself and release pancreatic enzymes including pancreatic lipase into serum. Measurement of serum concentration of pancreatic lipase can therefore aid in diagnosis of acute pancreatitis.<ref>"Pancreatic lipase". Wikipedia: The Free Encyclopedia. 7 Nov 2011 [http://en.wikipedia.org/wiki/Pancreatic_lipase]</ref>. Due to lipase's activity in the digestion and absorption of fat, there has been a growing market for lipase inhibitors for weight loss pharmaceuticals. The most popular is Orlistat (or Xenical®) which is a natural product from ''Streptomyces toxytricini'' and is the hydrogenation product of lipostation- an irreversible lipase inhibitor. This inhibitor also acts by binding Ser152, producing an ester which hydrolyzes so slow that it is practically irreversible <ref>Kordik, C., Reitz, A. "Pharmacological Treatment of Obesity: Therapeutic Strategies" Journal of Medicinal Chemistry, 1999 (42).</ref>. | ||
| - | |||
| - | </StructureSection> | ||
== 3D Structures of Lipase == | == 3D Structures of Lipase == | ||
| + | [[Lipase 3D Structures]] | ||
| - | + | </StructureSection> | |
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | + | ||
| - | **[[2es4]] – Lip+lipase chaperone C-terminal - ''Burkholderia glumae'' | ||
| - | }} | ||
==References== | ==References== | ||
<references /> | <references /> | ||
[[Category:Topic Page]] | [[Category:Topic Page]] | ||
Current revision
| |||||||||||
References
- ↑ [1] 1HPL PDB SUM
- ↑ [2] A cross-linked complex between horse pancreatic lipase and colipase
- ↑ [3] History of Lipids
- ↑ [4] 1HPL PDB
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=1HPL
- ↑ http://www.pdb.org/pdb/explore/remediatedSequence.do?structureId=1HPL
- ↑ http://www.springerlink.com/content/g5h1613440115701/fulltext.pdf
- ↑ Fundamentals of Biochemistry...
- ↑ Thomas, A. etc. "Role of the Lid Hydrophobicity Pattern in Pancreatic Lipase Activity", The Journal of Biological Chemistry, 2005 September 22; 270 (48): 40074-40083.
- ↑ "Colipase". Wikipedia: The Free Encyclopedia. 5 July 2011 [5]
- ↑ "Colipase Residues..."
- ↑ Fundamentals of Biochemistry...
- ↑ Crandall,W., Lowe, M. "Colipase Residues Glu64 and Arg65 Are Essential for Normal Lipase-mediated Fat Digestion in the Presence of Bile Salt Micelles" Journal of Biological Chemistry, 2001, (276) 12505-12512
- ↑ van Tilbeurgh H, etc."Structure of the pancreatic lipase-procolipase complex", 1992 Sep 10;359(6391):159-62. PMID:1522902.[6]
- ↑ http://www.pdb.org/pdb/explore/explore.do?structureId=1ETH
- ↑ http://www.nature.com/nature/journal/v362/n6423/abs/362814a0.html
- ↑ Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: a prototypic acetylcholine-binding protein. Science. 1991 Aug 23;253(5022):872-9. PMID:1678899
- ↑ Ollis DL, Cheah E, Cygler M, Dijkstra B, Frolow F, Franken SM, Harel M, Remington SJ, Silman I, Schrag J, et al.. The alpha/beta hydrolase fold. Protein Eng. 1992 Apr;5(3):197-211. PMID:1409539
- ↑ Bourne Y, Martinez C, Kerfelec B, Lombardo D, Chapus C, Cambillau C. Horse pancreatic lipase. The crystal structure refined at 2.3 A resolution. J Mol Biol. 1994 May 20;238(5):709-32. PMID:8182745 doi:http://dx.doi.org/10.1006/jmbi.1994.1331
- ↑ [7] 1LPB PDB SUM
- ↑ "Pancreatic lipase". Wikipedia: The Free Encyclopedia. 7 Nov 2011 [8]
- ↑ Kordik, C., Reitz, A. "Pharmacological Treatment of Obesity: Therapeutic Strategies" Journal of Medicinal Chemistry, 1999 (42).
Proteopedia Page Contributors and Editors (what is this?)
Michal Harel, Quinn R. Murray, Natalie Ziegler, Stephanie Schell, David Canner, Alexander Berchansky, Katelyn Clark, Eric Martz, Leben Tadesse, Joel L. Sussman, Eran Hodis